In the dynamic world of biopharmaceutical manufacturing, efficiency and compliance are paramount. The SciLog® NFF+ PF is an automated normal flow filtration (NFF) system that integrates seamlessly into downstream bioprocesses, supporting compliance with EU GMP Annex 1 (2022) for final fill and bulk filling applications.
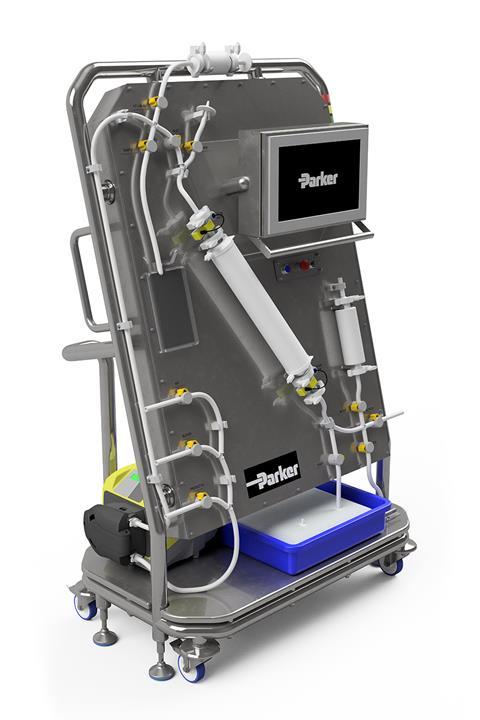
This multipurpose system features on-board Pre-Use Post-Sterilization Integrity Testing (PUPSIT), ensuring the integrity of filters before product filtration. Designed for GMP environments, the SciLog® NFF+ PF combines single-use sensing technology with automation to minimize user error. Its automated sequence monitors and adjusts pressure and flow rates, optimizing processing times and extending filter life.
Key features include:
- Configurable recipe creation for precise process control
- SIEMENS TIA Portal-based control system
- On-board integrity testing capabilities
- Compliance with FDA 21 CFR Part 11 and EudraLex Annex 11
- Touch-screen HMI with an intuitive interface
- Real-time communication of process parameters via OPC
- Optional integration of up to two scales for accurate filtration endpoints
- Redundant filtration variant (SciLog® NFF+ PFR) for enhanced safety
The benefits of the SciLog® NFF+ PF are significant. It includes manifold leak testing upon installation, PUPSIT validation before processing, and post-use integrity testing. This user-friendly system is designed to mitigate operator error, improving overall safety and efficiency.
Customization is also a key aspect, with the manifold designed to fit the system’s footprint, accommodating specific validated components. At Parker Bioscience, we understand the challenges of implementing PUPSIT, including retrofitting and cleanroom conditions. Our team is committed to supporting your integration needs.
In summary, the SciLog® NFF+ PF is more than a filtration system; it is a comprehensive solution that enhances bioprocessing capabilities, ensuring product integrity and operational efficiency in the biopharmaceutical industry.
For more information, visit the website here.