Realizing the Perfect Scale for Cell and Gene Therapies.
The explosive advancements within the cell and gene therapy sector have the driven the demand for high-quality GMP-compliant viral vector manufacturing. Adeno-associated virus (AAV) has been the primary vector driving this growth, as well as to a lesser extend lentivirus (LV) and adenovirus (AV). The use of these vectors is expected to grow fast also in the future and to diversify to include other therapies, such as oncolytic viruses.
The rapid growth and diverse use of viral vectors, present specific challenges in manufacturing for each potential therapy. The three main challenges are low titer to feed volume, upstream manufacturing in smaller scale and diversity of manufacturing conditions when compared to traditional biotherapeutics, such as monoclonal antibodies (mAbs). Also, the unoptimized hardware, consumables, and methods results in challenges with yield, purity, and cross-contamination.
The manufacturing scale of viral vectors is a lot smaller compared to mAbs. The pre-clinical manufacturing sizes range from hundreds of mL up to 20-50 L and early clinical phases from 50 L to 200 L, while the mAb production volumes have typically been over 500 L. As a result, hardware and consumables are not currently optimized to handle the smaller volumes with GMP-compliance. There is a technology gap existing for a small-scale, single-use chromatography system that allows closed processing, supporting the small volume needs coming from the viral vector and advanced therapeutics industry.
VIRAL VECTOR PURIFICATION OVERVIEW
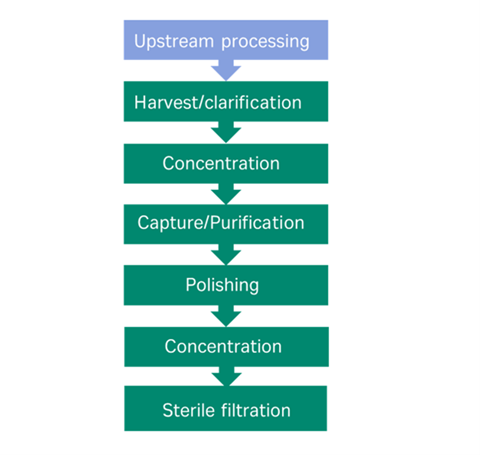
Despite the different physical characteristics between viral vectors, the current downstream processing protocols share similarities, with the goal of removing process impurities from the product. Typically, the workflow involves harvest/clarification (which may be proceeded by cell lysis depending on the vector), concentration of the bioreactor feed, capture/purification (density centrifugation or ion exchange/affinity chromatography), polishing (additional chromatography step/size exclusion) and finally sterile filtration, fill and finish (Figure 1).
One of the main challenges with AAV manufacturing is the separation of empty and full capsids. Full capsids contain the desired single-stranded DNA, while the empty ones do not. The separation is often demanding due to their small volume after affinity capture.
Purification of small quantities of virus for R&D, process development and/or small clinical studies has relied on density gradient ultracentrifugation, which can produce high purity and provide good resolution of full and empty AAV capsids, but the method is not considered suitable for manufacturing due to scalability limitations. Ultracentrifugation requires expensive equipment, high technical expertise, while the technique has volume limitations that is difficult to automate and scale for GMP manufacturing. From a regulatory perspective, the lack of system closure poses a greater risk of cross contamination particularly if many different vectors are produced in one facility.
In comparison, chromatographic separation is a more versatile, powerful, and scalable method for GMP environments to purify AAV. Notably, anion exchange chromatography with salt-step elution is one of the few methods capable of separating out the empty capsids for nearly all AAV serotypes.
SINGLE-USE TECHNOLOGIES
Closed system single-use technologies (SUTs) used for traditional biologics have been widely adopted for cell therapy and viral vector manufacturing for many of the same reasons: cost, time, and eliminating the need for cleaning validation, and systems cleaning procedures. This gives manufacturers the flexibility and agility to change over to different production campaigns without significant downtime, especially in multiproduct facilities. These improved process economies translate to higher productivity and safety.
Single-use chromatography systems offer many advantages over traditional systems for process intensification, but there are limited options commercially that are suitable for small volume purifications, which can complicate subsequent processing steps. For example, using chromatography systems and columns that are oversized for a small batch volume can result in high hold-up volumes that produce a product that is too dilute or being mixed with other liquids when processed, which makes it important to utilize a column that is correctly sized for the batch.
ÄKTA READY™ 450 PURIFICATION SYSTEM
Cytiva’s ÄKTA ready™ suite of single-use chromatography systems are designed for process scale-up and GMP manufacturing. The latest addition to this portfolio is the benchtop ÄKTA ready™ 450, which was designed specifically to meet the small volume purification needs of the cell and gene therapy industry (Figure 2). The system operates with ready‑to‑use, disposable flow paths in a closed system environment to mitigate the risk of contamination and cross-contamination of products.

Low hold-up volume and a low flow rate allows for optimal column residence time for accurate fraction separation. The integrated high-performing sensors in the flow path that include pH, conductivity and multi-wavelength UV provides users with real-time data without the need for manual sampling. UV absorbance (A260nm/280nm) detection is important in the separation of full and empty AAV capsids to achieve high purity.
The ÄKTA ready™ 450 system has been designed with ease of use in mind, allowing personnel of all skill levels to operate it.
The diversity of viral vector products that manufacturers need to accommodate in one facility has driven the evolution of clean rooms to smaller, modular designs, resulting in challenges using existing larger systems. The ÄKTA ready™ 450 has been designed with this challenge in mind with a compact, benchtop design allowing GMP-manufacturing in a smaller facility.
EASY TO SCALE
The entire ÄKTA™ ready system family is supported with an extensive documentation package for regulatory compliance well-suited for GMP environments and the intuitive UNICORN™ software, common across all ÄKTA™ platforms is also 21 CFR part 11 compliant. The UNICORN™ software is easy to use and permits connectivity between the systems for easy transfer of protocols and process parameters to easy scalability across platforms.

CONCLUSION
Too often in cell and gene therapy, there is a lack of fit for purpose manufacturing solutions and developers are forced to repurpose technologies designed for other biotherapeutics. This can lead to increasing costs and delaying these new therapeutics getting to market.
The launch of the ÄKTA ready™ 450 single-use chromatography platform system fills a gap in the portfolio for reliable and efficient GMP-purification at a scale suited for cell therapies and viral vectors, which can accelerate the release of material through the clinical stages to ultimately bring therapies to patients faster.
To learn more, please see: ÄKTA ready™ 450
Previously Published in Downstream Column
Copyright 2022 Downstream Column.
All Rights Reserved. www.DownstreamColumn.com