Leuven researchers combine polymers and zeolite in a membrane matrix for superefficient gas separation.
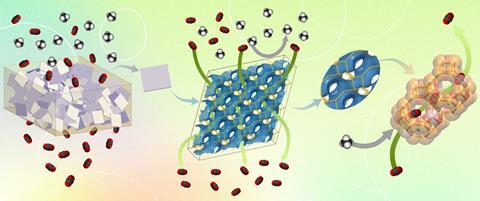
Separating CO2 from gas mixtures is an energy-intensive process. Zeolites, a type of molecular sieve, can help carry out this process. You can make membranes from pure zeolite, but that is difficult to do. In addition, zeolite membranes are not bendable and synthesis does not succeed for all types of zeolites. You can combine zeolite with a polymer to make a material that is easier to process, but if you mix a zeolite with a polymer in the traditional way, the adhesion between the two components is weak. The resulting membrane will leak and does not achieve the desired selectivity.
Researchers at KU Leuven synthesized a membrane that successfully combines the advantages of zeolites and polymers. They designed a mixed matrix membrane (MMM) of commercial polyimide filled with a zeolite. The MMM has a three-dimensional channel system that can accurately separate gas molecules. Their membrane outperforms all existing polymer membranes and most membranes made of pure zeolite. They published their results in Science in December.
’We achieve very high selectivity for separation of CO2 from a gas mixture of CH4 and N2 and at the same time high permeability,’ said Michiel Dusselier, co-author and catalysis professor at KU Leuven. ’The membrane structure can adsorb CO2, but also allows it to diffuse through it quickly. We can put up to 55% zeolite in the polymer, after which the membrane is still leakproof and flexible. Thus we create a kind of zeolite highway for CO2 in the polymer matrix.’












Nog geen opmerkingen