‘I know it won’t work, but I want to try it anyway’, Errikos Kounalis (Utrecht University) told his supervisor. In doing so, he unintentionally created a frustrated Lewis trio, a ligand-metal complex that had previously only existed as a theoretical concept in the literature.
Frustrated Lewis pairs (FLPs) consist of an electron-density accepting Lewis acid and an electron-density donating Lewis base that sit together in a sterically hindered, or frustrated, structure. Dissolving this frustration can catalyse chemical reactions; in 2006, the first FLP researchers used it to split hydrogen. Since then, the field has grown considerably and many (catalytic) reactions seem possible.
But Errikos Kounalis, a PhD student in Associate Professor Danny Broere’s group at Utrecht University, started out working on something completely different. During his master’s thesis – also with Broere – Kounalis developed a ligand to bring two metal atoms close together. ‘It was perfect for the later, “softer” transition metals according to the theory of hard and soft acids and bases’, Broere explains. ‘You can think of it as a ligand with two parking spaces where two soft metals could park.’
Strange
When Kounalis started his PhD, he had just returned from an internship in the USA. ‘I did a lot of chemistry with early transition metals like titanium.’ Broere was curious to see what this would do to the ligand, so Kounalis suggested he would just give it a go. Broere: ‘According to the theory, it shouldn’t work, but sometimes you just have to try things against your better judgement. Partly to test the theory, but also because you never know what might come of it.’
And so they tried. Kounalis complexed the ligand – t-BuPNNP with two coordinating phosphine groups – with the hard titanium and immediately saw something strange happen. ‘At the transition from one to two equivalents, the colour of the solution changed from aubergine purple to olive green’, says Kounalis. ‘When we looked at the crystal structure, instead of seeing two titanium atoms in the ligand, we saw a single titanium atom that had stolen a chlorine atom from another titanium complex in the solution.’
BMW
The two phosphine groups are supposed to coordinate to one metal atom each, but contrary to expectations, both sides shared the single titanium atom. ‘These Ti-P bonds were really very long, the longest known at the time’, says Kounalis. Broere adds: ‘Titanium behaved like a rude BMW taking up two parking spaces.’
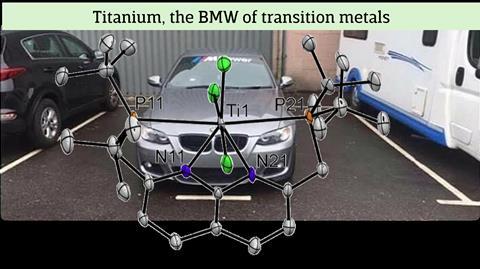
This discovery triggered the research now published in Chemical Science. ‘Because the phosphines cannot reach the titanium atom, there is a frustrated Lewis interaction’, explains Kounalis. ‘And because this frustration consists of three players, you can call it a frustrated Lewis trio [FLT, ed]. With this frustration, we then looked at reactivity. It soon became clear that we could convert epoxides, but exactly how this was done remained a mystery for a while.’
After a weekend experiment, he managed to isolate some crystals; the crystallographer said that the complex first converted the epoxide into an aldehyde and only then activated the aldehyde via the titanium-phosphine bond. ‘We thought we had a fairly complete story and were making good progress with the manuscript’, says Kounalis, ‘but when we delved into the literature we doubted whether we had the right approach for the article. It was only then that we saw that the existence of the Lewis trio had already been predicted in 2017, so we chose that as the focus of our paper and did a lot more calculations ourselves.’
Pendulum
The main difference between an FLP and an FLT is that the latter has an extra side arm. The researchers liken the interaction between the arms and the metal to a pendulum: two of the frustrated partners facilitate a reaction, while the extra side arm provides the necessary electron density by coordinating more or less to the metal.
‘The concept behind this reactivity, hemilability, can be seen in homogeneous catalysis, but also in enzymes’, explains Broere. ‘In a complex, a donor atom helps to create the best binding situation for a reaction intermediate by coordinating or not coordinating. The high activation energy of a chemical reaction is thus broken down into smaller steps, so that the reaction proceeds more quickly at a lower temperature.’ So with the motif from the paper, you have an extra handle to tune this kind of system to lower the activation energy even further. ‘It is the first time that hemilability has been demonstrated in an FLP-like system.’
Now that these fundamental and experimental steps have been taken, you can start to develop this further. Kounalis: ‘There is a lot to gain catalytically, you can easily vary the middle metal or the Lewis bases on the outside. The current complex should mainly serve as a springboard for further tunability.’
Kounalis, E. et al. (2024) Chem. Sci., DOI: 10.1039/D3SC06789K











Nog geen opmerkingen