A team of scientists from Twente was able to solve a hundred-year-old riddle concerning the formation of polyelectrolyte complexes (PECs) with NMR and are now able to measure and even predict what a PEC looks like on a molecular level.
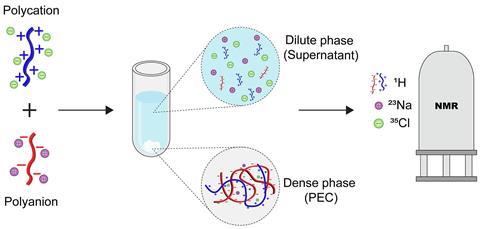
You may not have heard of polyelectrolytes, but their complexes (PECs) play important roles in biological processes and can serve as a basis for new materials. ‘Polyelectrolyte complexes form when you mix two solutions of oppositely charged polymers together’, explains Giulia Allegri, PhD student in the group of Associate Professor Saskia Lindhoud at the University of Twente. ‘A PEC system consists of two phases, a solid polymer-rich phase and a supernatant phase with a lower concentration of ions and polyelectrolytes. When the polyelectrolytes mix and form a PEC, they release their counterions.’ But the exact distribution of all the different components and how they interact has been a mystery since PECs appeared on the scene a century ago. Allegri, Lindhoud, and their colleagues Jurriaan Huskens and Ricardo Martinho pose a solution to this riddle in a recent article in the Journal of Colloid and Interface Science.
23Na and 35Cl
Their approach was simultaneously simple yet revolutionary. ‘Though people have used proton NMR to study PECs, we went beyond that with sodium and chlorine NMR to identify the counterions, which shows the whole picture and what forces are at play’, Allegri says. ‘How many counterions remain in the complex and how they interact with polyelectrolytes in the supernatant had not been studied before’, Lindhoud adds. ‘It appears that different ions have different effects, and we’re now able to measure these effects.’
‘We now have a complete molecular picture of the complex’
Saskia Lindhoud
The Twente team developed a method based on NMR with which they could quantify all the components in both the solid and the supernatant phase. For this study, they used poly(allylamine hydrochloride) (PAH, polycation) and poly(acrylic acid)-sodium salt (PAA, polyanion). ‘This approach allowed for determining relative and absolute concentrations of polyelectrolytes in both phases by 1H NMR studies. Using 23Na and 35Cl NMR spectroscopy we measured the concentration of counterions in both phases’, the authors wrote in the article. ‘NMR of both nuclei has been around for some time, but no one had used them yet for this topic’, says Allegri.
Stuck
‘With this method we found that the chloride ions interacted more with the polycation than the sodium ions with the polyanion’, Allegri says. It wasn’t all that easy to figure out, though. Lindhoud: ‘At first, we worked with collaborators to measure the ion content of the supernatant phase with ion chromatography. But the excess of polyelectrolyte released in this phase after complexation got stuck in the column, so we abandoned that approach and switched to NMR.’ The biggest challenge was the sample preparation, primarily due to the physical difficulty of separating the two phases, according to Martinho, who’s an expert in NMR at Twente. ‘We spent most of our time in that area, but as a result, we assured the accuracy of our measurements.’
This way, the team solved the whole mass balance and could sketch the number of bindings between polymers, how many groups there were and how many components were not charged. ‘In other words, we have a complete molecular picture of the complex’, says Lindhoud. Martinho elaborates: ‘Our findings help us to expand this work. Here, we used a binary system, but now we can also add a third component and understand or tune the system to our heart’s desire.’
Asymmetry
‘What was really surprising was that we discovered asymmetry in how the counterions bind to the polyelectrolytes’, Lindhoud says. ‘The polycation has a strong bond with chloride, but the polyanion was not as highly charged as we would expect.’ This means that the stoichiometry of polyelectrolyte to counterion is not 1:1. ‘The complex wants to be as close to electroneutrality as possible, however. We saw that PAA needed more protonation to fulfil the mass balance, indicating more PAH-chloride pairs in the complex.’
In this work, the researchers looked at weak polyelectrolytes. ‘Now, we’re moving to different structures like strong polyelectrolytes, which are always fully charged’, says Allegri. ‘I’ve already found some differences. Next, we’ll also try combinations of strong and weak ones, and there’s also the effect of salts on the polyelectrolyte complex formation. So there’s still a lot to discover!’
Allegri, G. et al. (2024) J. Colloid Interface Sci. 672, DOI: 10.1016/j.jcis.2024.06.062










Nog geen opmerkingen