Researchers in Wageningen have become the first in the world to create so-called catch bonds in the laboratory, which become stronger when force is applied to them. They report in the journal Nature Chemistry that this had previously only occurred in nature.
It started like so many things, begins Bauke Albada, associate professor at Wageningen University & Research (WUR). Researchers looked at nature and found something special: bonds that resist mechanical forces, called the catch bond. Normally, a bond weakens or breaks when pressure is applied, but in the case of the catch bond, the bond actually becomes stronger. ‘You can see this, for example, in white blood cells in the bloodstream, which need to be able to stick together in strong currents, but roll freely in slow currents’, says Albada.
Despite the fact that the catch bond is known to occur in nature, no one had managed to create it in the laboratory – until now. ‘It was up to the curiosity of the scientists to do it’, says Albada with a laugh. ‘Most papers have looked at this phenomenon at the single-molecule level, using an atomic force microscope to look at a single molecular interaction. But nature uses the bonds multivalently and at a macroscopic level, and that turned out to be the key to making the catch bond.’
Wrong parameters
Four years ago, Joris Sprakel – a professor of mechanobiology at WUR who had received an ERC Consolidator Grant to work on catch bonds – and Albada joined forces to get the research off the ground. But none of the many attempts was successful. ‘We also started at the single-molecule level, but we didn’t see the properties we needed because we were looking at the wrong parameters.’ They then looked at the applications mentioned in earlier work from other groups. ‘These contained many elements and were very complicated, but the step to the macroscopic level had to be made’, says Albada.
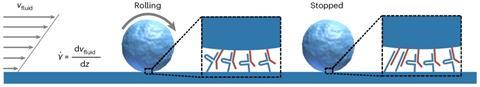
They did this as follows (see image above): they let small spheres flow through a microchip with tiny channels. On the surfaces of the channels and the spheres are small pieces of DNA. These are designed so that if they are complementary, they form a catch bond and stay put; if not, they roll on. With a special setup, you can read the interactions of the spheres with the channels and quantify their behaviour.
Hairpin
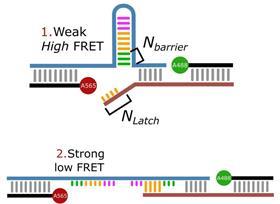
‘We designed it so that everything stays the same, except that you can play with the DNA strands’, Albada explains. ‘Our idea was to use a DNA hairpin for the catch bond. This hairpin can be pulled open like a zip, releasing a piece of single-stranded DNA.’ A small part of the released piece then binds to another piece of DNA that has a stronger interaction than the hairpin: the catch bond.
‘The complication was that our synthetic catch bond is slightly weaker than in biology. So we had to go to multivalent systems to measure it.’ Albada gives the example of a gecko sticking to a wall. ‘The Vanderwaals forces in a gecko’s foot are also not strong enough on their own, they only gain function when they work together, and the same goes for the catch bond.’
The star performers in this research were PhD student Martijn van Galen and master’s students Taieesa Peshkovsky and Annemarie Bok. Albada: ‘It was a very diverse project, but Martijn was able to get to grips with all of these things. Programming, computer modelling, organic and surface chemistry, building difficult setups, writing analysis scripts. He really did a fantastic job. On the other hand, the contributions of Annemarie and Taieesa were also crucial. They did the negative controls with so-called slip bonds, which behave a bit more classically. In these controls they discovered that in a macroscopic system you should not look at the force-lifetime, but at the behaviour of the spheres. Martijn then re-analysed the earlier measurements and then all the pieces of the puzzle fell into place. So it’s important to have several people to find the right angle.’
Ideal bonds
To top it all off, they also found ‘ideal bonds’. ‘That was a really nice coincidence’, says Albada. ‘Between catch bonds and slip bonds, we saw properties that fit the ideal bond, which always has the same interaction regardless of external forces. Unfortunately, we didn’t develop it further due to lack of money, but knowing that others were looking for it, we made our claim anyway and it was accepted.’
‘Now that we’ve figured out how to make them, the real fun can begin’, Albada continues. ‘You can fine-tune the catch bonds or shape them differently and start looking for applications.’ Together with his colleagues, he is already thinking about materials that have a certain fluidity under normal mechanical stress, but become firmer instead of weaker as the stress increases. ‘Hydrogels are a good example. They already incorporate DNA for things like drug release. If you want to integrate something like that into the body, they should become firmer when force is applied, but not permanently.’
According to Albada, it is particularly important for this type of project to have a talented jack-of-all-trades. ‘You need someone who wants to keep going, who is not afraid of new steps and who has the energy to try new things. Only with that combination of commitment, skill and talent can you see something like this through to the end. We almost gave up several times, but we managed to keep believing in what we were doing.’
Van Galen, M. et al. (2024) Nat. Chem. DOI: 10.1038/s41557-024-01571-4













Nog geen opmerkingen