With a dual-chamber system, you can use carbon monoxide to install a carbonyl group during synthesis in a relatively safe way, a Flemish team shows in the European Journal of Organic Chemistry.
Usually chemists shy away from using carbon monoxide in organic synthesis because of the high toxicity and flammability of the gas. But if you can control those properties, you have a reactive carbonyl group in hand that offers numerous synthetic possibilities. Sarah Vangrunderbeeck, Wim De Borggraeve and colleagues from KU Leuven found an application for a reagent mix they previously devised to generate CO outside the reaction mixture (i.e. ex situ).
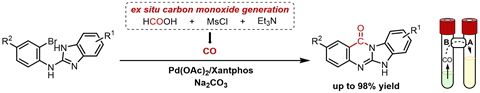
Ex situ
The group used a two-chamber reactor for which the basis lies in Denmark, at the research group of Troels Skrydstrup (University of Aarhus), states first author Vangrunderbeeck, currently a PhD student at both the KU Leuven and UCLouvain. ‘In one room you let formic acid react with mesyl chloride and tri-ethylamine to release CO gas. Via an air bridge, the gas can diffuse to the other chamber and be installed there as a carbonyl group.’
You can screw the reactor completely shut, so that no CO gas escapes. ‘But you have to remain watchful’, Vangrunderbeeck continues. A possible leak – even in reactions on a small scale — can have dramatic consequences and you want to detect it in time. ‘That is why we pin a CO detector to our lab coats, to minimise the risks.’

OLED screens
With the system, the Leuven team made derivatives of benzimidazo[2,1-b]quinazolin-12-ones. Quinazolinones in general exhibit numerous biological functions’, Vangrunderbeeck explains. ‘Because they are so planar, some types of quinazolinones can insert themselves into DNA and thus prevent DNA replication in tumour cells.’ Other scientists also found that benzimidazoquinazolinones are possibly immunosuppressive. This offers leads for the treatment of autoimmune diseases.
But the molecules are not only biopharmaceutically interesting. Vangrunderbeeck: ‘The most likely application is in electronic materials, because they are electron-rich molecules. In a patent, I read about a possible application in OLED screens. However, our research was mainly concerned with the new synthesis methodology.’
‘We pin a CO detector to our lab coats to minimise risks’
Carbonylation takes place in the last of the two reaction steps. First, you make N-(2-bromophenyl)-1H-benzimidazol-2-amines with various side groups. Once you have these molecules, you can do an intramolecular palladium-catalysed carbonylation with CO. ‘The largest scale on which we did the reaction was one gram, but the yield was only 55%’, the PhD student says. ‘The biggest challenge in this whole process is not necessarily in the CO incorporation, but rather in the purification, because the product is often difficult to dissolve in conventional solvents.’
Another thing you can do relatively easily with this two-chamber system is to label molecules with 13C isotopes. Instead of normal formic acid, you take formic acid with a carbon 13 atom. From that, you make 13CO that you can incorporate into the heterocyclic ring; that way, you get a labelled product.

Other gases
In the same way, you can generate and use other (dangerous) gases, says Vangrunderbeeck. In 2017, De Borggraeve’s group already published about the use of the two-chamber reactor for the ex situ generation of sulphuryl fluoride (SO2F2), and earlier this year in Chemical Science about trifluoromethane sulphonyl fluorides (CF3SO2F). These are gases with which you can do ‘Sulfur(VI) Fluoride Exchange (SuFEx)’. With SuFEx chemistry, a new generation of click reactions has been added to organic synthesis. To make optimal use of this, a safe way to make SO2F2 or CF3SO2F is very convenient, concludes Vangrunderbeeck. ‘I expect that with this system you can use many more gases in synthesis. Getting access to such gases in a simple way at lab scale is very useful within this field.’
Vangrunderbeeck, S. et al. (2022) EurJOC 2, doi.org/10.1002/ejoc.202101136
Li, B-Y. et al. (2022) Chem. Sci. 13, doi.org/10.1039/D1SC06267K
Veryser, C. et al. (2017) Org. Lett. 19(19), doi.org/10.1021/acs.orglett.7b02522











