Researchers at the University of Liège have succeeded in producing bio-based ruthenium catalysts from caffeine and theophylline. Their method, published in the journal Organometallics, should make the production of these widely used catalysts greener.
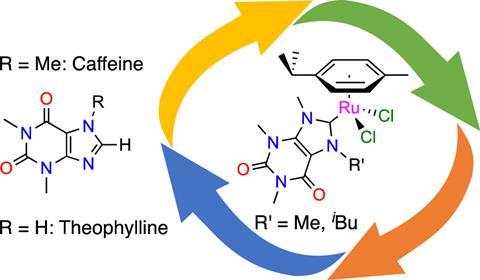
Catalysts are usually synthesised from non-renewable raw materials such as oil and metals. Chemists are looking for ways to reduce the carbon footprint of these processes. François Mazars and Lionel Delaude, researchers at the University of Liège, have used caffeine and theophylline as precursors of biological origin to synthesise two ruthenium catalyst precursors. These provide effective catalysts for three common organic reactions: the transfer hydrogenation of unsaturated substrates with isopropanol, the oxidation of alkenes with sodium periodate and the synthesis of vinyl esters from 1-hexine and benzoic acid. They published their results in the journal Organometallics.
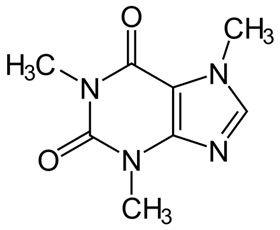
Caffeine and theophylline are both xanthines, or molecules with the same central ring structure as xanthine, and are found in coffee beans, tea leaves and cocoa beans. They are easy to obtain: all that is needed for the extraction and separation processes is water and supercritical CO2. The researchers used these compounds as N-heterocyclic carbene precursors (NHC precursors) and then converted them into two ruthenium-arene complexes with the general formula [RuCl2(p-cymene)(NHC)].
According to the researchers, their proof-of-concept study shows that a labile p-cymene ligand and an NHC ligand from caffeine or theophylline can be combined to efficiently prepare a range of highly active ruthenium catalysts.
Mazars and Delaude (2023) Organometallics https://doi.org/10.1021/acs.organomet.3c00166













Nog geen opmerkingen