Alkyl groups around a carbon atom destabilise it, making the resulting carbocation relatively more stable. This fact is unjustly overlooked, write Dutch theoretical chemists in Chemical Communications. At the same time, they point out the danger of using isodesmic reactions.
Carbocations are reactive intermediates in organic chemistry that you see frequently in fundamental synthetic reactions. These reactive particles are generally formed by breaking a C–X bond, for example carbon-halogen compounds such as R3C–I or R3C–Cl. Until now, it was thought that this C–X bond became weaker if you added more and more alkyl groups to the carbon atom, because this would increase the stability of the carbocation. ‘That is certainly not incorrect,’ says Thomas Hansen of the Vrije Universiteit Amsterdam. ‘It just doesn’t give the complete picture, there is more behind it.’ Hansen published the complete picture in Chemical Communications with Pascal Vermeeren, Matthias Bickelhaupt and Trevor A. Hamlin, all three affiliated with the same university. Recently, the article even made the cover.

Hansen and his colleagues show that the more important effect takes place in the starting molecule: by adding more and more alkyl groups (in this case methyls) to R3C–X, it becomes less stable. That is what drives the stabilisation of the carbocation, which the group believes has been completely overlooked so far. ‘The hyperconjugation between the cation and the substituents is also important, but it is not the main contributor,’ Hansen stresses.
To confirm that repulsion in the starting molecule causes the instability of the C–X bond, Hansen did calculations on a thermodynamic cycle of dissociation from R3C–X to R3C+ + X– (see scheme below). ‘In the calculation, you first break off the substituents of the central carbon atom,’ he explains. ‘Then you can compare how the enthalpy changes when you add more and more alkyl substituents, i.e. methyl groups in this case. You then do that for both the starting molecule and the carbocation. Our comparison showed that the starting molecule destabilises and the carbocation actually stabilises. But the stabilisation factor in this case is less than the destabilisation factor.’
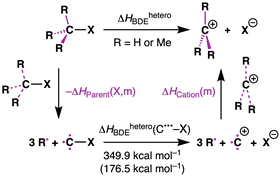
Trevor A. Hamlin points to their recent research on radical stability (which we wrote about earlier): ‘The methyl groups actually did not stabilise the radical formed after cleavage of the C–X bond at all, but caused instability. The carbocation does get stabilised by the methyl groups, but that is not the leading term.’
Danger of isodesmic reactions
Why this factor was overlooked for so long is because of the use of isodesmic reactions. These are reactions in which you have the same number of bonds of the same nature before and after the reaction, for example C–C, C–H and C–X bonds, as in H3C–X + R3C+ → H3C+ + R3C–X. You then calculate the bond enthalpies before and after the reaction and then put them side by side. Hamlin: ‘What many people and textbooks overlook is that in isodesmic reactions, more than one particle determines the stability of the system. Teachers and researchers tend to focus only on the reactive, “more interesting” particles in these isodesmic reactions and thus overlook the “duller” particle that in this case has the decisive role in the system.’
Matthias Bickelhaupt also confirms this. ‘Isodesmic reactions are common because they are quite easy. In the lab, you don’t just pull a methyl group off a carbon atom; you don’t need to in such an equation. The method is not wrong in itself, but its interpretation is, especially the assumption that trends and effects only come from the ions. That misunderstanding needs to be cleared up.’ Bickelhaupt also mentions that this is often not considered at all. ‘In papers you either see nothing about this, or something incorrect is implicitly assumed, or the wrong assumption is even explicitly mentioned. And then the interpretation necessarily goes wrong.’
‘This is why we welcome Thomas’s calculations,’ Hamlin adds. ‘It used to be very difficult to calculate through such a thermodynamic cycle, but Thomas has managed to do it in a simpler way. Now we can incrementally look for the right bonds.’
Hansen, T. et al. (2022) Chem. Commun. 58, 12050-12053, doi.org/10.1039/D2CC04034D












Nog geen opmerkingen