NADPH dehydrogenase can not only reduce but also oxidise by simply raising the pH, as researchers from Delft show in ChemCatChem.
Enzymes are biology’s organic chemists. No wonder that some chemists want to use these reaction machines to carry out chemical reactions. One such enzyme family that is already moving towards industrial applications is NADPH dehydrogenase, also known as Old Yellow Enzyme (OYE).
Traditionally, OYEs have been used for the reduction of conjugated C=C bonds. However, there were already indications that the reverse should also be possible, so the groups of Frank Hollmann and Caroline Paul decided to find out to what extent OYEs could be used for oxidation.
Article continues below the image
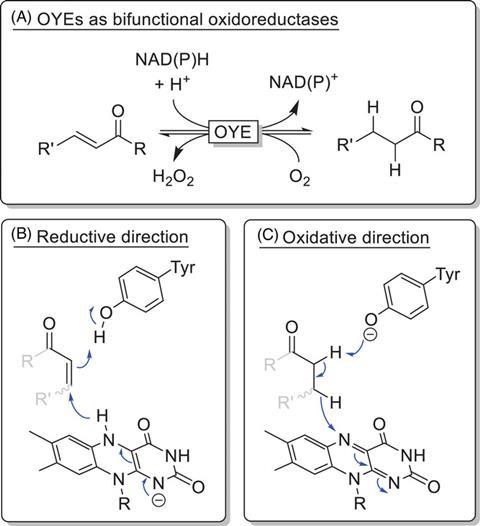
Flavin mononucleotide (FMN) is attached to the OYEs. This structure can initiate the reduction step by using its hydride in a type of Michael addition. OYE properly completes the reaction by protonating the resulting enolate via a tyrosine in the enzyme.
It turns out that this tyrosine plays a key role. OYEs are normally used at a pH between 6 and 8, where the tyrosine remains protonated. But at a higher pH (here 8.5), it loses its proton, allowing it to act as a base and initiate the oxidative reaction of the OYE, where FMN recaptures the hydride.
The researchers applied this reaction to a variety of substrates and found, as expected, that the same substrates that would normally undergo reduction were also oxidised. There are probably many more possible substrates, but they limited themselves to a few standard examples to show the principle. Another nice thing about this reaction is that you don’t really need to heat it up: it works fine at 30 °C.
Finally, they wanted to find out whether OYEs – other than the OYE from the bacterium Thermus scotoductus that they studied – also oxidise when the pH is raised. A number of OYEs stood out as showing considerable activity and could therefore be investigated further. In any case, a new oxidation tool for biocatalysis with OYEs has been confirmed.
Van Hengst, J.M.A. et al. (2024) ChemCatChem e202401447, DOI: 10.1002/cctc.202401447











Nog geen opmerkingen