
Electrochemistry may be an underexplored option for recycling plastic waste, researchers show in a review in Chemical Science.
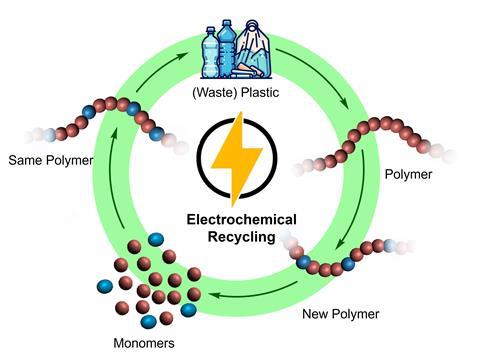
Plastics have been revolutionary for almost all aspects of human life. Imagining a society without polymer-based materials would be quite the challenge. But the inert nature of most of these polymers poses a problem: what do you do with the accumulated waste? Weizhe Zhang, Lars Killian and Arnaud Thevenon from Utrecht University explore the recent advancements in the relatively new field of electrochemical recycling of polymeric materials in their review.
The beauty of these electrochemical methods is that they should be applicable to both current and future polymers. ‘Electrons are traceless reagents’, says Assistant Professor Thevenon. ‘By adjusting their potential energy, you could in theory selectively depolymerise mixed polymer wastes, assuming that the downstream purification of the products is not too energy intensive.’
PVC
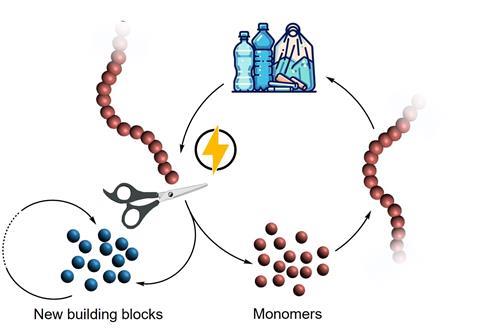
In the review, the authors discuss several techniques and compare them to traditional recycling methods. With electrochemistry, you can break up certain polymers to their respective building blocks; functionalise polymers electrochemically; or do paired electrocatalysis, in which you oxidise or reduce a polymer while doing the opposite to another molecule.
‘Modifying polymers this way makes them applicable to more traditional methods of recycling, like reuse, and mechanical and chemical recycling’, says Zhang, postdoc in Thevenon’s group. Thevenon adds: ‘There was one study where researchers recycled PVC electrochemically. They discovered that you can use the additive that is already present in the material as a redox mediator. Usually, you have to add that to your reaction mixture, but here they used something that was already present in the waste.’
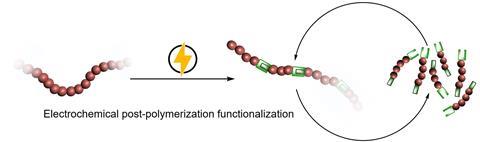
Adding specific functional groups to a polymer is also an interesting option, Thevenon continues. ‘The introduction of functional groups into chemically inert polymers such as polyolefins may enable us to introduce weak linkages into the polymer chains without altering the properties of the material. Those weak linkages could later be used to trigger depolymerization back to monomers.’
Replace
Though the options sound promising, the electrochemical way will not replace the traditional options. ‘We discuss the traditional methods a lot, since the electrochemical method is still only two or three years old’, says Zhang. ‘And since there may be significant differences between waste streams, it’s better to combine all the techniques.’
Electrochemical recycling will be a complementary method, Thevenon agrees. ‘I don’t think it’s even possible to chemically recycle all plastics, it’s too energy intensive. We should first look at mechanically reusing and repurposing, and use chemical recycling as a last resort, in which electrochemistry will fulfil a niche role.’ That doesn’t mean it doesn’t deserve attention, says Zhang. ‘With our article, we wanted to encourage people to try more electrochemistry and to give them something to work with.’
The first step towards broader implication would be to push the available knowledge to a higher level, according to Zhang. ‘First, we have to determine what waste streams we have, like which types of plastic produce the most waste or are most difficult to recycle’, he says. ‘Then, we need to increase our knowledge of the field and the possibilities, and also set up more collaborations with industries. And at the beginning of the plastic making process, we need to design better reactors that take recycling into account.’
Dirtiness
‘Another important step to take is doing fundamental studies in polymer problems’, says Thevenon. ‘Polymers are long molecules and often insoluble, so the field has to develop methods to either do electrochemistry in a heterogeneous mixture or develop new electrolytes or new ways to dissolve the materials.’ The dirtiness of plastics must also be considered. ‘Electrochemistry is usually done with pure feedstock, but that’s not the case with waste plastics. So we need to find systems that can deal with this.’
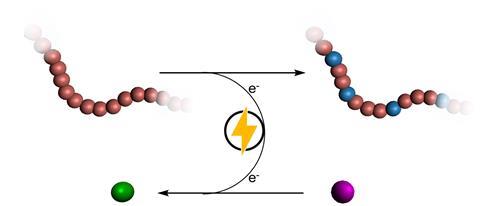
Thevenon suspects that it might not be efficient enough to apply electrochemistry to waste plastics. ‘Maybe you should only use it to convert pristine plastics into other types of plastics. This is where the post-polymerisation functionalisation strategies could prove very useful. With it, you can create polymers that you cannot make in any other way.’ Thevenon gives the example of polyethylene. ‘If we can oxidise them to polyesters, chemical recycling would become much easier. Another possibility is to overoxidise them with electrochemistry to CO2 and maybe some other elements, which you could then purify and reuse to make new polymers from. But that’s all very energy intensive.’
In any case, there remains a lot of research to be done. ‘Our article is based on techniques that are developed for small molecules’, Zhang explains. ‘So we need to explore if these methods work the same way for bigger molecules, or if we should broaden the research so that it becomes applicable to polymers. We hope to stimulate the community to develop new strategies and solutions to tackle these issues.’
Zhang, W., Killian, L. and Thevenon, A. (2024) Chem Sci 15, DOI: 10.1039/D4SC01754D











Nog geen opmerkingen