Selective poisoning of the cathode catalyst surface with CO increases the efficiency of NO reduction to NH3 in polymer electrolyte membrane cells. Delft researchers show this in ChemSusChem.
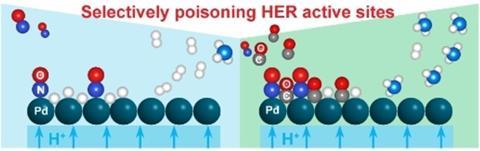
A catalyst that performs better by partially poisoning the surface? A group of researchers at TU Delft has succeeded in doing just that. They worked on the reduction of NO to NH3 in a polymer electrolyte membrane cell (PEM) in which Cu/C, Pd/C and Pt/C, among others, act as catalysts. In this system, NO reduction competes with the hydrogen evolution reaction (HER). However, when they added CO to the feed, they found that it poisons the surface in such a way that it selectively suppresses the HER, increasing its efficiency towards NH3. They published their results in ChemSusChem at the beginning of August.
Ammonia is one of the most produced inorganic substances in the world. About 80% of it ends up in fertilisers, making ammonia an essential substance for the world’s food supply. We currently produce ammonia using the Haber-Bosch process, which requires high temperature and pressure. An alternative production process, ideally based on green electricity, would save a lot of energy and CO2 emissions. Direct electroreduction of NO to NH3 is promising, according to the researchers, partly because the process can be run continuously.
Cu/C was initially the most active catalyst for NO electroreduction, with a so-called Faraday efficiency of 78% at 2.0 V. Pd/C and Pt/C had higher efficiencies for hydrogen. In general, HER dominated at high cell potential. When CO was added to the feed, the efficiency to hydrogen was significantly lower for all catalysts because CO competitively and selectively binds to the active sites for HER. This effect was most pronounced for Pd/C. This improved the surface occupancy of NO, which increased the efficiency towards NH3.
Li et al. (2023) ChemSusChem https://doi.org/10.1002/cssc.202300949











Nog geen opmerkingen