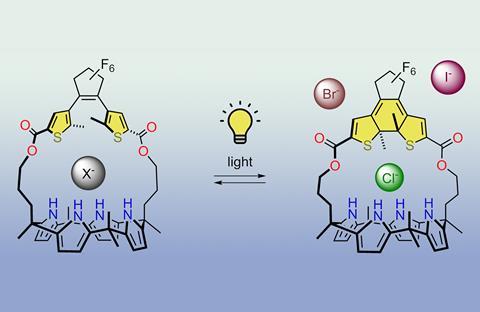
By building an anion receptor with a gap that can be widened or narrowed by shining light on it, you can specifically control which anion binds, Leiden researchers write in Chemical Communications.
Imitating nature is one of the specialities of chemists. Anion receptors are no exception. Making them yourself has obvious applications in sensing and transport. One popular synthetic anion receptor is calix[4]pyrrole, because of its strong affinity for halogen ions. Researchers in Sander Wezenberg’s group at Leiden University have created a light-activated variant: one isomer allows all kinds of halogens to enter, while the other keeps larger ions at bay.
The modification they gave to calix[4]pyrrole was a molecular ‘strap’ of dithienylethene. Among other things, it is made up of three five-rings that join together to form a hexagon under the influence of light. The loop version, without the hexagon, provides an opening where calix[4]pyrrole can use its halogen affinity to bind halogen ions such as chloride, bromide or iodide.
When exposed to light at a wavelength of 300 nm (ultraviolet), the hexagonal isomer forms and the gap becomes smaller. Chloride is not significantly affected, but its affinity for bromide is reduced by a factor of 160, the largest change in affinity ever observed for this type of receptor. Light with a wavelength of 630 nm (red) is needed to break the six-ring (and allow bromide and iodide back in).
‘One of the possible future applications could be in sensing’, says Wezenberg. ’Not necessarily as a sensor itself, but as an extractant in cases where background noise would prevent quantitative analysis. You could then selectively extract the analyte, use light to completely separate it from the receptor and then quantify it.’
In an NWO-M1 project, Wezenberg’s group is looking at the extraction of cesium chloride/bromide ion pairs. ‘Calixpyrrole is selective for caesium salts’, he explains, ’and this could be interesting for cleaning radioactive waste water. As this is quite an expensive technology, you could justify using a slightly more complicated – and therefore more expensive – molecule.’
But the group is not there yet. Wezenberg: ’At the basic level, we are developing the technology to control the binding affinity with light, which is a big challenge. But the difference in affinity for bromide between the light-switchable states described in this paper is the largest compared to what has been achieved so far. So the met-molecular switch-bridged calix pyrrole receptors seem to be well suited for controlling the binding properties.’
Villarón, D. et al. (2023) Chem. Commun. 59, DOI: 10.1039/D3CC02264A












Nog geen opmerkingen