Ben Feringa’s group has found a simple and versatile way to make molecules in which the chiral centre sits on the phosphorus atom. They explain the possibilities in Angewandte Chemie.
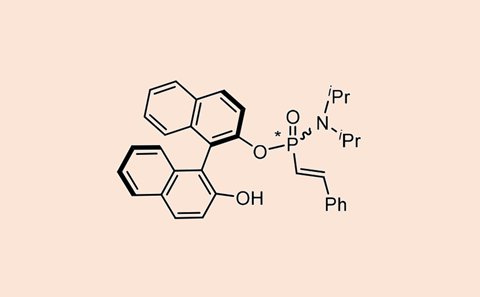
Molecular structures with a chiral phosphorus atom appear in many areas of research, from bioactive and pharmaceutical compounds to ligands in asymmetric catalysts. But making them is not so easy, although some progress has been made recently. Xiao-Bing Chen, Damián Padín, Charlotte Stindt and Ben Feringa from the University of Groningen have now developed a method for the stereoselective synthesis of alkenylphosphonamidites, which you can bend to your will with minor adjustments to the reaction conditions.
The beauty of the method is that you can work with commercial or readily available substances, in this case phosphoramidite variants and halogen alkenyls. You combine the phosphoramidite with a haloalkenyl using the commercially available catalyst Ni(cod)(dq), with the phosphorus changing from the P(III) oxidation state to P(V). And this is where the first tweak starts: if you add Cs2CO3 as a base, you get the opposite diastereoselectivity than if you add another base or no base at all.
Similarly, all kinds of tweaks are possible. For example, for a phenylalkenyl with two bromides, one on the alkenyl and one on the phenyl: if you do the reaction with a palladium catalyst and Cs2CO3, the phosphorus atom couples on the phenyl side, but if you use the nickel catalyst without base, the alkenyl side couples. The researchers also carried out the first stereoselective addition to the salt of an alkenyl phosphonamidite (to their knowledge) and used the alkenyl phosphonamidite as a modular building block for the synthesis of phosphorus stereogenic compounds.
Chen, X. et al. (2023) Angew. Chem. Int. Ed. e202307450, DOI: 10.1002/anie.202307450












Nog geen opmerkingen