If you want to produce paracetamol (acetaminophen) or ibuprofen in a sustainable way, use β-pinene from biowaste streams as a starting point, suggest UK researchers in ChemSusChem.
Many substances are still derived from fossil sources. As governments and companies increasingly move away from fossil sources, scientists are looking for alternative sources. One such sustainable source can be found in forestry or the citrus juice industry. The waste streams from these industries contain limonene and turpentine, a mixture of monoterpenes such as α-pinene, β-pinene and 3-carene. They can be used as building blocks for everything from medicines and fragrances to biofuels and polymers. Joshua Tibbets, Steven Bull and colleagues at the University of Bath wanted to lead by example and developed a scalable synthesis of paracetamol and ibuprofen using β-pinene as a starting point.
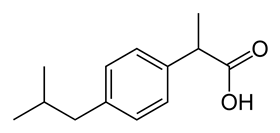
The plan was to produce an intermediate first, after which the route would split towards paracetamol and ibuprofen. From β-pinene, the researchers made 4-isopropenylcyclohexanone (4-IPEC) via oxidation, acid-catalysed ring-opening and dehydration (removal of an H atom) on a twenty-gram scale. From 4-IPEC, paracetamol and ibuprofen are produced in three and five steps respectively. Both synthesis routes are characterised by palladium-catalysed hydrogenation, which plays an important role in making the ring aromatic. In this way, they showed that β-pinene can serve as an excellent starting material.
On the way to paracetamol, the researchers came across the intermediate 4-hydroxyacetophenone (4-HAP). This intermediate, they reasoned, could also be used as a building block for other sustainable aromatic products. Not only does 4-HAP lead to paracetamol, but the compounds dyclonine (oral anaesthetic), metropolol (beta-blocker), salbutamol (bronchodilator) and the microbicide BHAP are also accessible from 4-HAP.
Bull and colleagues did not investigate the synthesis routes for these latter compounds, but rather mentioned them to inspire other researchers and show what is possible with a terpene biorefinery. They hope that success of this study will inspire others to design their own sustainable routes from biorenewable feedstocks to industrially important benzenoid products that are currently sourced from non-sustainable petrochemical feedstocks.
Tibbetts, J.D. et al. (2023) ChemSusChem e202300670, DOI: 10.1002/cssc.202300670














Nog geen opmerkingen