Painters in the seventeenth century used their paint to create the highly convincing illusions. The discovery of unusual arsenic sulfide pigments in The Night Watch and other paintings from the same period by researchers from the Rijksmuseum, the University of Amsterdam and the University of Antwerp, indicates that their palette was even more varied than previously thought.
Lieutenant Willem van Ruytenburch is certainly one of the most eye-catching figures in Rembrandt van Rijn’s Night Watch. Part of the central pair of officers in the painting, he is bathed in light, as if put in a spotlight. He stands out because of his clothing, particularly his fashionable, sleeveless buff coat trimmed with dense embroidery in gold thread. Even now, almost four centuries later, that embroidery still seems to shine.
As part of Operation Night Watch, the large-scale research project into Rembrandt’s monumental painting that the Rijksmuseum started in 2019, researchers Fréderique Broers and Nouchka De Keyser discovered that Rembrandt used a special pigment for his golden glow. They published their results in the journal Heritage Science. Broers and De Keyser are doing PhD research at the University of Amsterdam, the University of Antwerp and the Rijksmuseum on pigments and paint degradation in seventeenth-century paintings and the imaging techniques that can be used to study these.

Arsenic
An X-ray fluorescence scan (MA-XRF) of The Night Watch had revealed the presence of arsenic in several places, including parts of Van Ruytenburch’s clothing. De Keyser: ‘That could indicate smalt, a blue pigment Rembrandt used a lot, but in these spots in the clothes we did not find the other elements you usually associate with that pigment, such as cobalt and nickel. We therefore took two minuscule paint samples at these spots so we could better analyze the pigment particles.’ Under the electron microscope, they turned out to be compounds of arsenic and sulfur. It was suspected that they were orpiment (As2S3) or realgar (As4S4), two toxic arsenic sulfides that painters used as respectively yellow and red-orange pigments.
[Article continues below]
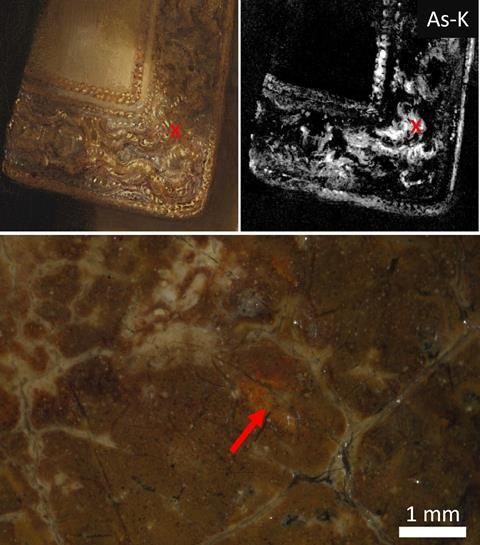
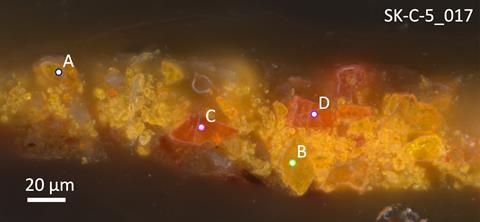
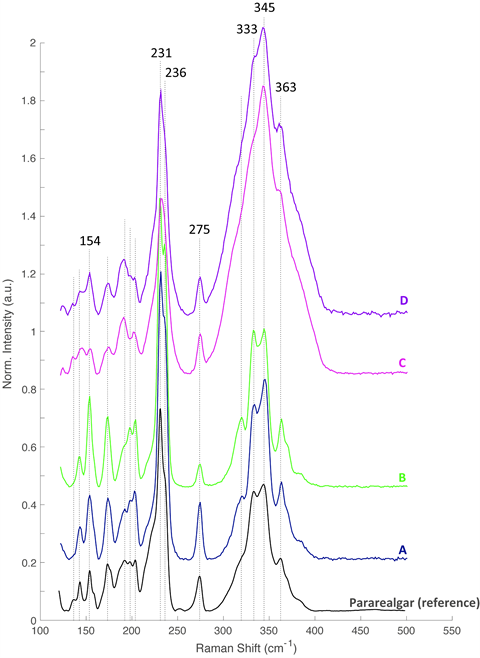
But some questions remained, as the observed particles had a shape that differed from natural arsenic sulfide particles and from arsenic sulfide particles that have been observed in until now only two other paintings by Rembrandt. Using Raman spectroscopy, the researchers demonstrated that these were less common arsenic sulfides: the yellow pararealgar, an isomer of realgar, and a semi-amorphous pararealgar that is reddish orange in color.
Oranges
Pararealgar is usually known as a degradation product of realgar formed under the influence of light, but the researchers had no reason to believe that they were dealing with degraded paint layers. ‘The pigment particles looked intact’, Broers explains, ‘and the electron microscope image showed no obvious degradation activity. Moreover, you would expect the degraded, yellow particles closer to the surface, where they come into contact with light, but that was not the case either.’
Pararealgar has previously been observed in paintings and researchers have hypothesized that in these cases it could have been the original pigment, not degraded paint. ‘Because arsenic sulfides have been so little researched, it remains difficult to judge in general whether it is a product of in-situ paint degradation or the original pigment. But it seems that various artists used it as a yellow pigment, although they probably bought it under the name orpiment.’
[Article continues below]

Had Rembrandt made this particular mixture himself, in order to best imitate the shiny gold embroidery? To investigate this, the team took a close look at several other seventeenth-century paintings from the Rijksmuseum’s collection. ‘In a still life by Willem Kalf, painted some 20 years after The Night Watch, we found a paint layer in one of the depicted oranges that looked almost exactly the same as the paint sample from Van Ruytenburch’s buff coat, with similar peaks when analyzed with Raman spectroscopy. This most probably means that the mixture was sold in this specific composition in Amsterdam during that period.’
Specialized lab
Broers believes the research could be a starting point for a broader rediscovery of ancient pigments: ‘In a so-called collector’s cabinet with miniature apothecary in the Rijksmuseum’s collection, there are at least 25 arsenic sulfides. If you only imagine orpiment and realgar as arsenic sulfide pigment, you might well ask: why are there 25? I truly think that a lot of knowledge about those specific pigments has disappeared over time, and that by using modern techniques we can start to distinguish them again.’ De Keyser confirms that the historical picture is probably richer: ‘In the German sources, you find a lot more different names and recipes for these pigments. They were possibly traded, and sold abroad, under more generic names.’
‘This research has demonstrated that it is really necessary to do additional analyses to pinpoint what type of arsenic sulfide you are dealing with,’ says De Keyser. In particular Raman spectroscopy is a technique that provides clarity in that respect. ‘You want to know why the artist used this particular pigment, and not a natural arsenic sulfide. And you want to step into the shoes of those that made the pigments, to try out different recipes. Would it be possible to produce that semi-amorphous form of pararealgar we have found in the paint sample, for instance? And how do these different pigments degrade? The problem is that you would need a specialized lab because these are highly toxic substances. Hopefully someone will take on the challenge.’
Nouchka De Keyser, Fréderique Broers, et al., Discovery of pararealgar and semi-amorphous pararealgar in Rembrandt’s The Night Watch: analytical study and historical contextualization, Heritage Science (2024), doi:10.1186/s40494-024-01350-x













Nog geen opmerkingen