
A team from Leiden has developed an extremely potent antibiotic against Gram-positive bacteria, including drug resistant strains, as reported in a recent study published in Science Translational Medicine.
Readers of C2W | Mens & Molecule will undoubtedly be familiar with antimicrobial resistance and the threat it poses to humanity, with projections of 10 million people dying each year by 2050 due to resistant bacterial infections. Luckily, chemistry has many tools in the toolbox to find solutions for the many types of resistance. To this end Emma van Groesen, Nathaniel Martin and colleagues from Leiden University found a molecular modification that can be made to the clinically used antibiotic vancomycin that results in exceptional activity.
Vancomycin is often a ‘last resort’ antibiotic, but this molecule too suffers from increasingly resistant bacterial strains. ‘In this case, we’re talking primarily about Gram-positive bacteria as opposed to Gram-negative’, says Van Groesen, who obtained her PhD in 2022 and is currently a scientist at a biotech company in Utrecht. Martin, Professor of Biological chemistry, adds: ‘Resistance in both types of bacteria are serious, but looking at sheer numbers, the Gram-positive ones are more common.’

Derivatives
The new antibiotic designed by the team is directed at Gram+ and has been some time in the making. ‘One of the first synthetic steps even stems from Nathaniel’s own postdoctoral research, which I was able to reproduce at the start of my PhD’, Van Groesen recounts. ‘It shows that ideas you might work on at the beginning of your career can linger in the background and might be useful later on.’
‘A few years before Emma started, our group was working on a different project involving the synthesis of compounds containing substituted guanidine [HNC(NH2)2, ed.] moieties, which are fully protonated at physiological pH’, Martin explains. ‘At the time we developed a nice chemical method to introduce the guanidine group into different molecules and showed that we could also substitute it with different lipid tails.’ In her PhD, Van Groesen then further elaborated this concept and applied it to make a whole range of novel vancomycin derivatives.
‘Holistic’
One of the compounds with a guanidino lipid group attached to the vancomycin – called EVG7 as it was Van Groesen’s seventh such analogue – is extremely active, so much so that the team sometimes jokingly called it the ‘holistic antibiotic’, since the effective concentration is hundreds-to-thousands fold lower than that of vancomycin itself. ‘On top of that, the compound is also effective in overcoming vancomycin resistance. I’m afraid the antibiotics we develop in our lab have a much higher standard to live up to now’, Martin says with a laugh.
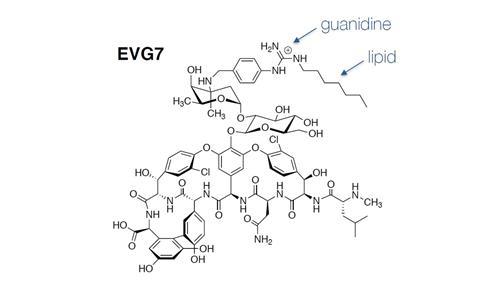
He explains that introduction of the lipidated guanidino group to vancomycin is so effective because of the high positive charge it carries. ‘As I mentioned, guanidine is fully protonated at physiological conditions, which gives rise to its charge. What’s unique to Gram-positive cells is that their surface is negatively charged. That’s why the positively charged guanidine has a high affinity for the cell wall.’
But that’s not the whole story. ‘The lipid tail attached to the guanidine group also functions as an anchor in the bacterial membrane and has to be just the right length’, Martin continues. ‘If it’s too short, there’s no antibacterial activity, and if it’s too long it becomes toxic to mammalian cells.’ Van Groesen adds: ‘The compound is active against both bacterial strains containing the standard D-ala-D-ala version of the bacterial cell wall building block lipid II, as well as those using the D-ala-D-lac version associated with glycopeptide resistance.’
Waiting
They submitted their manuscript to Science Translational medicine in 2022 and then the biggest ‘challenge’ for Van Groesen began: ‘I really don’t mind being busy in the lab, but waiting for our work to be published? Very hard!’ she says laughing. It was for a good reason, though. ‘We sent it to the highest impact translational medicine journal, so we expected a lot of feedback and extra work, which was fine’, says Martin. ‘But in the end, it took nearly 2,5 years for it to be published.’ All-in-all, they have been very fortunate, Martin concludes. ‘The researchers involved were great, the funding was available, and we also had a bit of luck here and there. There’s never a guarantee that ideas on paper will also work in the lab or beyond.’
Market
In an article on our website from 2022 about the state of antibiotics (in Dutch), Martin said that ‘the current market doesn’t make antibiotics R&D attractive for companies to invest in’, which would make the future development of the drug uncertain. ‘But in the years since then there have been some promising changes on the reimbursement side’, he says.
‘Health ministries and others are beginning to see the importance of antibiotics, and an environment has been created in which companies can make some money with antibiotics’, Martin elaborates. ‘For example, there are now trials being undertaken involving so-called “subscription models”, in which there is a guarantee that companies will be paid if they produce innovative new antibiotics, irrespective of the number of prescriptions written.’ The difficulty with marketing antibiotics is that a very effective antibiotic will not immediately be used but is seen as a last resort option only to be used after all other antibiotics have failed. ‘The subscription model would decouple that, giving companies a profit while they provide the drug on an as-needed basis.’
With this in mind, Martin is hopeful for the future of EVG7. ‘There is interest from pharma companies that might want to license our compound.’ It will be interesting to follow EVG7’s journey to see where it will end up.
Van Groesen, E. et al. (2024) Sci Trans. Med. 16(759), DOI: 10.1126/scitranslmed.abo4736











Nog geen opmerkingen