A team from Groningen and Barcelona cleared up what takes place when you put a designer enzyme with unnatural amino acids through a directed evolution campaign, as shown in ACS Catalysis. ‘We didn’t anticipate this at all.’
Enzymes are magnificent catalysts, but they are usually also hyper specific, meaning they can do one chemical transformation extremely well. This also comes into play when designing new enzymes. ‘Another limitation is that there’s only so many reactions you can do with natural enzymes’, says Reuben Leveson-Gower Assistant Professor at TU Delft. ‘In the lab of Gerard Roelfes – where I did my PhD – they initiated an interesting strategy to expand the reactions.’ Roelfes’s group uses unnatural amino acids in their enzyme designs. ‘Encoding these gives new chemical functions.’
During his PhD, Leveson-Gower used para-aminophenylalanine (pAF) to imitate organocatalysts. ‘Organocatalysis is very useful for chemical syntheses and of course won the Nobel Prize in 2021. This type of chemistry could be really powerful and efficient if done in an enzyme, and that’s exactly what we accomplished.’
Promiscuity

Leveson-Gower’s predecessors, Ivana Drienovská and Clemens Mayer, initially developed the artificial enzyme with pAF and Mayer led a directed evolution campaign to optimize the active site side chains. However, when he took over the project at the start of his PhD, Leveson-Gower investigated the promiscuity of the artificial enzyme, that is, its ability to catalyse two very different reactions. ‘The results weren’t perfect, though, so I did my own directed evolution campaign’, he explains. ‘But as we did, we saw that this process caused the enzyme to be specialised and lose its promiscuity, much like what happens with natural enzymes. That’s why we set out to figure out what was causing this and we published the results in this paper.’
Together with colleagues from Barcelona, the Roelfes group combined mutagenesis, computational chemistry and structural biology to discover the underlying mechanism. ‘It was quite a big team effort with collaboration between disciplines, and it was really nice to work together with people from a range of different backgrounds.’
Completely different
The team had two important and rather unexpected findings. ‘Some mutations caused the protein itself to change shape in an unexpected way, which we didn’t anticipate at all’, says Leveson-Gower. ‘We expected that mutations surrounding the active site would change the polarity or something like that. But they changed how the protein dimer came together.’ The active site lies in between the two subunits, and the mutations rearranged the quaternary protein structure, which gave a completely different effect.
Another remarkable observation was that the mutations that the team introduced had a strong so-called epistatic effect. ‘The individual mutations showed but little improvements in our enzyme, but combining multiple mutations led to a much greater effect’, Leveson-Gower explains. ‘We think that the mutations created a new and improved hydrogen bonding pattern which stabilized the active site. When we removed one hydrogen bond donor and added a new one, we could steer the reaction pathway in a new direction.’
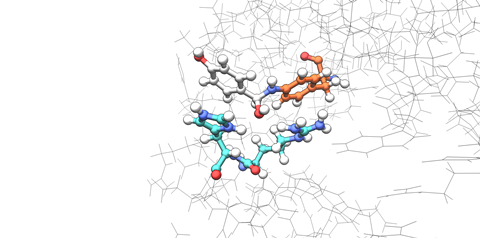
According to the assistant professor, this also shows that just a small number of mutations can cause radical changes in the function of a designed unnatural enzyme. ‘We see that the unnatural components we use can strongly cooperate with the natural amino acids that the surrounding protein is made out of. We hoped for this to be true, but now we have the evidence to show that it really happens.’
Bashing
The project wasn’t always smooth sailing, however. ‘I had to learn how to be a computational chemist from scratch during this project’, Leveson-Gower reminisces with a smile. ‘I did the majority of the computations and learned most of the techniques during an internship in Barcelona.’ There was a lot of troubleshooting, ‘bashing my head against the wall’, but overall it was very beneficial. ‘It was a nice learning opportunity and also very valuable to do research in another country; it gives a different perspective.’
What’s the takeaway of this work? Leveson-Gower: ‘What we’ve characterized here is a manner in which unnatural amino acids can cooperate with natural amino acids to boost the efficiency of catalytic reactions. It can serve as a motif for others to follow.’ To make the best out of directed evolution, you should follow-up with a mechanistic study to see what has happened exactly. ‘This way we move more and more towards rational approaches for enhancing unnatural enzymes.’
Leveson-Gower, R.B. et al. (2025) ACS Catal. 15(3), DOI: 10.1021/acscatal.4c06409











Nog geen opmerkingen