If you put gold nanoparticles in a graphene shell, you can see the molecules on the gold surface with an electron microscope, even in a liquid. This is what a Flemish-Spanish team reports in Nature Chemistry.
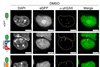
Gold nanoparticles have already been widely studied and applied, but less is known about what happens on the gold surface with the ever-present ligands. Indeed, electron microscopy (EM) allows one to see the nanoparticles very well, but small, light elements such as carbon are difficult to visualise. ‘In addition, most EM research is carried out in high vacuum, which is not a realistic environment for these molecules’, says Sara Bals, professor at the Department of Physics at the University of Antwerp. Bals’ group, in collaboration with Spanish researchers, therefore decided to make it even more challenging by looking at these surface molecules in liquid using transmission EM (TEM).
They did this by making ‘envelopes’ out of graphene. ‘We trapped the liquid with the gold nanoparticles and ligands between two layers of graphene’, Bals explains. ‘The advantage of graphene is that it is just a one-dimensional layer of carbon, which gives a negligible background signal in the TEM. In addition, the good thermal and electrical conductivity of graphene ensures that the electron beam causes virtually no damage to the ligands.’ The sheath structure limits the amount of liquid between the layers so that it does not further interfere with the already weak signal from the surface molecules. ‘It’s technically very well done and leads to new insights in materials science.’
Chiral
These findings are mostly fundamental. In fact, the structure of surface molecules was not entirely clear in the literature. The research of Bals and her colleagues has greatly improved this understanding. ‘But now we can go further’, says Bals enthusiastically. ‘We are now developing chiral nanoparticles. In other words, we’re looking how to transfer chirality from molecules to inorganic materials.’
Bals shows a picture of a nanoparticle that is not straight but twisted, almost like a DNA helix. ‘Our Spanish co-authors can now grow this kind of chiral nanoparticle by using chiral surface molecules’, Bals continues. ‘But how exactly does this happen? That is the next step for the methodology we have now published. If you understand the growth of these particles, you can better control the chirality, which in turn leads to stronger interactions between chiral inorganic materials and chiral organic molecules.’
Highlight
The work demanded a lot from the team. ‘A lot of steps had to be optimised and a lot of patience was needed to fine-tune each parameter. So I have a lot of admiration for the people who do the research in the lab.’ For Bals it was particularly special to capture a micelle of surface molecules moving around a nanoparticle and becoming part of the ligand shell. ‘Being able to capture that moment was really a highlight.’
The fact that Nature Chemistry has published the research makes Bals very happy. ‘It’s the perfect conclusion to my ERC Consolidator Grant project RealNANO, which I submitted in 2018. In it, we wanted to study nanomaterials under realistic conditions.’ Instead of the artificial conditions usually found in an EM – that is, room temperature and a high vacuum – Bals looked at materials in the gas environment at high temperature. ‘I was quick to decide not to use a liquid environment because of the difficulty; we were already treating the nanoparticles with a plasma cleaner, so we didn’t have to think about the ligands.’
Gradually, however, the idea to visualise the ligands became stronger. ‘But you can’t measure real ligand structures in a vacuum, you have to measure them in liquid’, she said to herself. ‘It was also the last part of the project, so we really had to do it to make it complete. Even though it was much more difficult than we imagined, I am very happy that it all worked out.’
Pedrazo-Tarjados, A., Claes, N., Wang, D. et al. (2024) Nat. Chem. DOI: 10.1038/s41557-024-01574-1











Nog geen opmerkingen