Using a biphenyl crown ether, you can read the programmed stereochemistry in a molecular tape like a kind of Turing machine, British researchers show in Nature.
David Leigh’s group from the University of Manchester compares the way a cell processes information to a Turing machine. Such a machine reads sequences of information and assigns a value for each character read. This should also be possible at the molecular level, Leigh must have thought. In any case, the publication in Nature shows something similar.
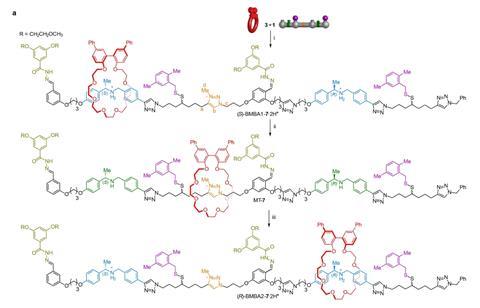
Leigh and his colleagues Yansong Ren, Romain Jamagne and Daniel Tetlow designed a ‘nanomachine’ (as they call it) that you power with acids, bases and some small molecules. The machine consists of a molecular tape with different reading points, and a reading head in the shape of a crown ether.
If you make a solution with both components, you can use the acidity to control the machine. Add trichloroacetic acid in excess to the solution and the crown ether will wrap around the first acidified fragment of the strand. The acidification also changes other bits on the tape so that the ring stays in place and cannot go back, just like a ratchet. If you then add triethylamine, the ring can slide on to the next fragment. Then you can push the crown ether forward one more time by adding acid and the ring will eventually slide off the strand when you add base again, after which you can repeat the process.
Attached to the crown ether is a so-called quaterphenyl, which can take on different stereochemical conformations depending on the fragment it surrounds. You can read that conformation with circular dichroism (CD) spectroscopy. Tapes with different stereochemistry get a different code that way. Tape 1 from the paper had an S conformation at the first fragment, then a neutral one and an R conformation as the third. This then gives the code -1, 0, +1. Tape 2 had the same sequence but had an S conformation at the last position, resulting in -1, 0, -1.
Although this is still relatively simple, Leigh and colleagues foresee that based on this system you could, for example, change the stereochemistry of a catalytic chemical reaction so that the “molecular ratchet can transcribe information from one form to another by reading the tape”. This, according to the authors, approaches close to ribosomal transcription that resembles ‘Turing’s original description of an automatic machine that can do arbitrary calculations.’
Ren, Y. et al. (2022) Nature, doi.org/10.1038/s41586-022-05305-9











Nog geen opmerkingen