Researchers have succeeded in recycling powdered aramid fibres using microwave radiation, according to a paper in the Journal of the American Chemical Society.
The Closing Carbon Cycles with Renewable Amines (3CRA) consortium aims to explore how valuable amines can be extracted from biomass and recycled. ‘Amines are important building blocks for the chemical industry’, says Vincent Voet, lecturer in circular plastics at NHL Stenden University of Applied Sciences. ‘They can be obtained from residual streams, but also from plastics and materials such as the aramid fibres in Twaron.’ Together with colleagues, Professor Katja Loos’ group at the University of Groningen and the company Teijin Aramid (which produces Twaron), they joined forces and pooled their expertise to see if the aramid fibres could be recycled in this way.
‘It is actually a kind of KNCV paper’, says Loos with a laugh. ‘Bert Gebben is a senior scientist at Teijin Aramid, but also chairman of the KNCV Macromolecules section. Vincent is treasurer and Joël Benninga, the PhD student who carried out the research, is the committee’s webmaster. I am also President of the KNCV Soft Matter Section. So we know each other well and already had a good basis of trust for this project.’ The extensive cooperation between the university and the college is also something special. ‘It doesn’t happen very often. This more practical approach and collaboration should be seen more often at universities, we need to focus more on societal challenges.’
Fifteen minutes
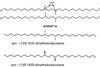
Aramid fibres have many applications, including cut-resistant gloves, wear-resistant tyres and bulletproof vests. But recycling is still in its infancy, and once the material is worn out, it is usually downcycled. Voet: ‘Theoretically, you could put the fibres straight into the microwave, but we started with the powder form of the polymer, poly(p-phenylene terephthalamide).’ With some water and NaOH in the microwave at 250°C, the polymer hydrolysed into the monomers p-phenylene diamine and (a salt of) terephthalic acid in fifteen minutes.
Once the polymer had been successfully broken down into the monomers, the main challenge began: how do you get them pure? ‘Joël was looking for the right way to separate them from each other and from the residual materials’, says Voet. ‘This is always a challenge in recycling: depolymerisation often goes well, but purification requires a lot of research.’ Benninga found success with a mixture of extraction and crystallisation.
‘When you are considering a process for industry, you have to think about these practical steps’, says Loos. ‘It works in the lab, but how will it work on a large scale?’ Voet adds: ‘Teijin Aramid, of course, has a lot of experience with the chemistry behind aramid fibres and is keen to take steps in the field of recycling. That is why the company got involved in the process at an early stage. This is what I like about this kind of public-private partnership.’
Radiation
There are still a few unanswered questions. ‘In particular, what is the degradation mechanism?’ says Voet. Loos: ‘Simply heating in an oil bath is much slower, so what exactly happens during depolymerisation?’ A number of papers that have looked at microwave reactions seem to suggest that the microwave radiation itself influences the process, so that is what the researchers want to find out. In science, one question always leads to another’, says Voet.
The next step will be to recycle real fibres instead of powder. ‘Then you look at fibres with additives and so on, with more and more pollution’, explains Loos. ‘It is a high-tech application, especially compared to other plastics such as PET. Once the technology is developed, it is up to the industry to do something with it.’ The researchers are confident that the process has a future.
Criticism
One criticism from the industry might be that aramid fibres are bad for the environment. ‘But most products that contain aramid can be properly collected, so they do not end up in the environment. Nevertheless, you have to be careful what you use each type of plastic for’, says Loos. ‘You have to be aware of how you use plastics because you never know in advance whether a material can cause damage to the environment.’
‘Also, as a researcher, I don’t really believe that science is that black and white. I don’t believe that there is, for example, a single best fibre or a single best way to recycle’, adds Voet. ‘This is similar to the polarisation you see in society as a whole. We are not claiming to have the best solution, but if you want a sustainable future, you have to answer these kinds of questions [about recycling and environmental impact, ed.]. As researchers, we need to work with our partners to provide the pieces of the circular puzzle of the future.’
Benninga, J. et al. (2025) JACS 147(9), DOI: 10.1021/jacs.4c17791











Nog geen opmerkingen