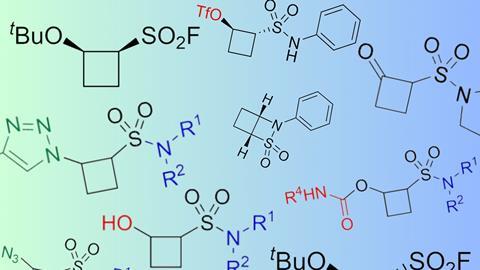
1,2-substituted cyclobutanes can be easily prepared under high pressure and are interesting building blocks for drug development, a team from Nijmegen shows in the European Journal of Organic Chemistry.
In addition to the synthesis of complete drugs, chemists are also involved in so-called fragment-based drug discovery. This involves the production of small (reactive) fragments that can be used as ‘building blocks’ in the molecular design of drugs, allowing the rapid testing of specific structures. The group of Floris Rutjes (Radboud University), together with colleagues from Symeres and VU Amsterdam, synthesised a small library of what they considered to be an underrepresented structure: 1,2-disubstituted cyclobutanes.
It is no coincidence that they focused on these square structures. Previously, the Rutjes lab had developed a method for performing [2+2] cycloadditions, where high pressure is required to achieve this particular reaction. They have now applied this strategy to tert-butyl vinyl ether and ethylene sulphonyl fluoride (ESF). In this process, the substituents of the resulting cyclobutane end up side by side on the molecule (the 1- and 2-positions). The best results were obtained using MeCN as the solvent, NaHCO3 as the base and a pressure of 12 kbar.
They then modified this cyclobutane by making differently substituted sulphonamides from the sulphonyl fluoride and deprotecting the tert-butyl to form the cyclobutanol. The resulting molecules in turn formed the basis for a total of 55 cyclobutane variations, including azides, triazoles and carbamates. The researchers are now investigating the biological properties of the compounds.
Janssen, M.A.C.H. et al. (2024) EurJOC e202400797, DOI: 10.1002/ejoc.202400797











Nog geen opmerkingen