Australian researchers have succeeded in synthesizing zinc crystals in a solution of liquid metal, resembling snow crystals.
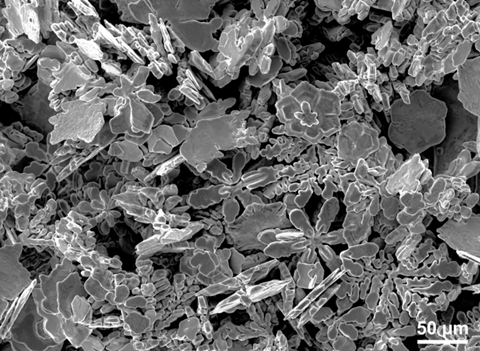
Snowflakes are ice crystals that grow in different shapes, but always with sixfold symmetry. Zinc crystals can grow similarly in a solution of liquid gallium, Australian researchers report in Science. They found a new method for extracting these metallic crystals from the liquid metal, then were able to study the morphology and symmetry of the resulting crystals. At sufficiently high temperatures, metals such as zinc easily dissolve in liquid gallium. When the solution cools again, the zinc crystallizes. The researchers suspected that these metallic zinc crystals would exhibit diverse morphology, but the high surface tension of liquid gallium made it impossible to extract the crystals without damaging the microscopic structure.
Shuhada Idrus-Saidi (University of New South Wales) and colleagues still managed to extract the zinc crystals from solution by lowering the surface tension of the liquid metal via an electric field. In addition, they used vacuum filtration to separate the liquid metal from the crystals. This produced highly symmetrical crystal structures that look a lot like six-pointed snowflakes. The researchers then synthesized further crystals from other metals and solutions, including silver, nickel, copper and platinum, which produced yet other crystal shapes. This general method makes it possible to create highly crystalline and fine metallic structures from liquid metal solutions.











Nog geen opmerkingen