With a well-designed iron catalyst, it is easy to make Suzuki-Miyaura couplings, US researchers show in ACS Catalysis.
Catalysis must be efficient. That is why transition metals such as palladium, platinum and rhodium are in high demand. The only drawback is that they are relatively rare and sometimes toxic. Catalysis with iron would be ideal, as it is one of the most abundant (and therefore cheapest) metals. Paul Peterson, Paul Chirik and colleagues at Princeton University have now achieved this with the Suzuki-Miyaura coupling. Normally, this involves using palladium catalysts, but by tweaking them, you can use iron as well.
Something like this has been tried before, but it required a water-sensitive and non-commercially available amide base. With a few modifications, the Americans were able to use the common and mild base potassium ethoxide (KOEt). The iron catalyst consists of Fe(IV) surrounded by pyridine, CH2SiMe3 and a phenoxyimine. If you put 10 mol% of this into your reaction equation, it catalyses a whole series of Suzuki couplings.
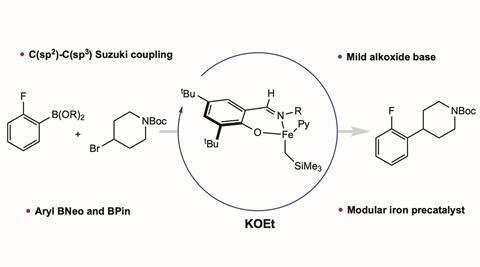
In this case, the Suzuki reaction couples arylboric acids to alkyl bromides. In this case, electron withdrawing aryl groups reacted better than electron neutral or electron donating groups. Variation on the alkyl bromide side was also possible, even with base-sensitive functional groups. This is not possible with Grignard bases or alkyllithium or amide bases, but the use of the alkoxide KOEt allows base-sensitive functional groups on both parts of the coupling.
Because of the relatively wide range of reaction conditions, the iron catalyst may also be applicable to pharma-relevant reactions, the authors conclude.
Peterson, P.O. et al. (2023) ACS Catal. 13, doi.org/10.1021/acscatal.2c05838











Nog geen opmerkingen