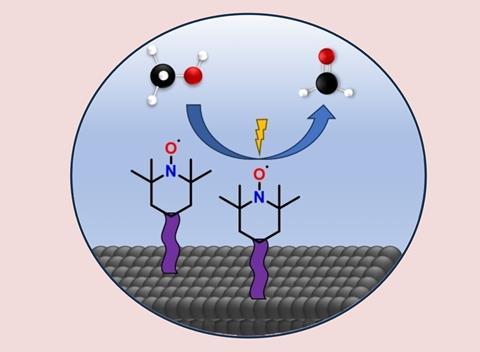
If you attach the TEMPO catalyst to an electrode, it can replace the role of platinum in the oxidation of methanol to formaldehyde, as researchers from Amsterdam show in ChemSusChem.
The chemical industry is increasingly relying on electrification for the energy transition. Or at least it wants to. This is because not all thermocatalytic processes are equally easy to convert to electrocatalytic variants. One example is the oxidation of methanol to formaldehyde. If you want to do this electrochemically, you need expensive platinum or silver catalysts. Or do you? Pim Broersen, Joost Koning, Gadi Rothenberg and Amanda Garcia from the University of Amsterdam have developed an organic catalyst with higher efficiency and excellent selectivity.
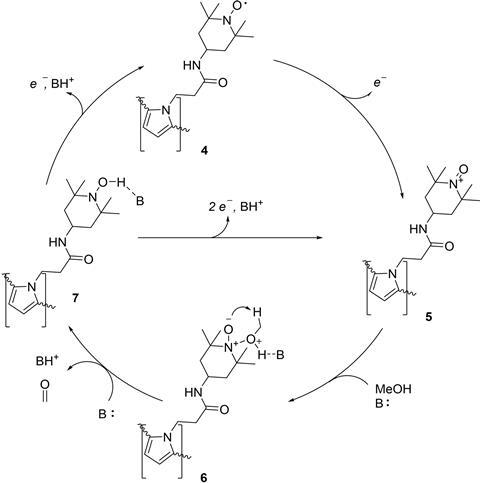
The researchers used the well-known alcohol oxidant TEMPO (2,2,6,6-tetramethylpiperidinyloxyl), a radical that is regularly used as a homogeneous catalyst in organic chemical reactions. One advantage of TEMPO is that it prefers to oxidise alcohols rather than carbonyl groups. they grew a polymer film on the support electrode and anchored TEMPO to it with a pyrrole group. This makes TEMPO a heterogeneous catalyst.
The next step is to oxidise methanol. For this reaction, however, you need a base, and the researchers tried a whole range of weak and strong bases. Using lutidine as a weak base, they achieved a Faraday efficiency of 97.5%. Although this is very high, the turnover number (TON) was quite low, only about 1250. With the stronger base CsOH, the efficiency was slightly lower (70-85%), but the TON shot up to 17,100.
They also tested whether they could oxidise other alcohols with their setup, and experiments with 1-propanol and isopropanol showed that this was also a possibility. One caveat to the research, however, is its small scale. ‘We used small electrochemical cells of ten millilitres’, says Amanda Garcia, associate professor of synthesis, catalysis and electrochemistry, in an email. ’This is normal for proof-of-principle work, which is where our research falls.’
If you want to work with this reaction on an industrial scale, you have to overcome some obstacles. ‘In industry, you usually need much higher current densities and thousands of hours of reaction time’, says Garcia. ‘And one challenge with that is the stability of the TEMPO-modified electrode, but with flow chemistry you might be able to get around those kinds of challenges.’
‘In any case, our approach could have a big impact on the sustainability of formaldehyde production’, continues Garcia. ‘After all, with TEMPO we carry out the reaction at room temperature, without a precious metal catalyst, and potentially using green electricity.’ Another advantage is that TEMPO is relatively cheap, at around €60 per kilogram. ‘Using TEMPO then gives a big advantage over platinum or silver, but first we need to do some work on the stability of our catalyst.’
Broersen, P.J.L et al. (2024) ChemSusChem e202400582, DOI: 10.1002/cssc.202400582












Nog geen opmerkingen