
An international team has developed a synthetic strategy to make NCF3, SCF3 and OCF3 anions for pharmaceutical applications with far fewer PFAS-generating steps, Science reports. The strategy is timely, given the upcoming tightening of regulations on fluorine-containing molecules.
There is currently a love-hate relationship with fluorinated molecules. Rightly so, of course: PFAS pollution is having a major impact in some places. At the same time, fluorine is an essential element in many medicines and agrochemicals.
A recent article in Chemical & Engineering News considers how we should look at fluorinated medicines: are they PFAS or not? In 2021, the Organisation for Economic Co-operation and Development (OECD) published a new definition of PFAS, expanding the scope to include ‘fluorinated substances containing at least one fully fluorinated methyl (-CF3) or methylene (-CF2-) carbon atom’. These types of compounds are common in medicines and in the substances used to make medicines containing fluorine.
If this definition were to lead to legislation, other ways of making these types of molecules would have to be developed. An international team led by Timothy Noël, professor of flow chemistry at the University of Amsterdam, was already working in this area when the OECD proposal was published. ‘We immediately saw that our method could make an important contribution to reducing PFAS waste while maintaining access to the benefits of CF3 groups in drugs’, Noël says in an email. ‘As the article [from C&EN, ed.] clearly shows: without fluorine, it is very difficult to develop effective drugs; this would probably lead to other side effects.’
Noel and the team have developed ‘a unique concept that can be generalised to the three main fluorinated moieties: NCF3, SCF3 and OCF3’, he explains. They do this without PFAS starting materials, but with cheap organic precursors and the salt cesium fluoride in a flow system. ‘This way, you just make the reagent on site and you can use it immediately, significantly reducing the number of steps that generate PFAS waste.’
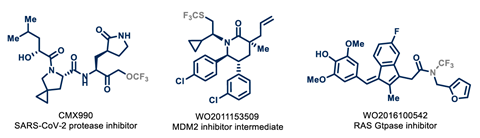
For all three CF3 structures, there is a similar protocol: let the precursor react in flow with CsF – in the presence of a crown ether to trap the caesium – and then let it flow into a separate batch or flow reactor with the electrophile you want to attach the CF3 group to. Thus you only need one fluorine source: CsF. As electrophiles, they have used both simple molecules, natural substances (such as cholesterol and androsterone) and existing drug intermediates (such as ibuprofen and celecoxib).
But does this not leave you with a PFAS-like group, in this case CF3? ‘I have two responses to this’, writes Noël. ‘Firstly, drugs and agrochemicals need these groups. If you leave them out, the products are less effective, which may mean that larger quantities are needed, or that they have to be applied more often on the land. That is not desirable either.’ Noël’s second point is that when CF3 groups are next to a heteroatom, there are metabolic pathways that can break them down. ‘In other words, these are not persistent chemicals.’
So, together with the fact that flow chemistry also makes it a scalable method, this strategy offers hope for ‘cleaner’ fluorinated drugs.
Spennacchio, M., Bernús, M., Stanić, J. et al. (2024) Science 385(6712), DOI: 10.1126/science.adq2954











Nog geen opmerkingen