If you coat the inside of a microfluidic channel with poly-L-lysine, you can use it to influence signals in chemical reaction networks, says ChemSystemsChem.
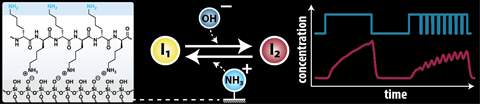
Equilibrium is essential: (bio)chemistry is full of it. In nature, it is still a little more elegant than what you can produce in the lab, but that does not stop chemists from creating equilibrium systems themselves. Hazal Koyuncu, Jacopo Movilli, Sevil Sahin, Dmitrii Kriukov, Jurriaan Huskens and Albert Wong at the University of Twente have created such a system, which will ultimately allow information to be processed at the molecular level using chemical reaction networks (CRNs). This has earned them a place on the cover of ChemSystemsChem.
Wong and colleagues used a 3D-printed microfluidic channel through which they could pass different concentrations of molecules and then read the signals. ‘We wanted to know what happens when you pass equilibrium systems through such a channel and how you can influence them’, says Wong, associate professor of chemical reaction networks. ‘One way to do this is to attach a layer of charged molecules to the inside of these channels. In our case, this was poly-L-lysine, an amino acid that has a positively charged amine.’
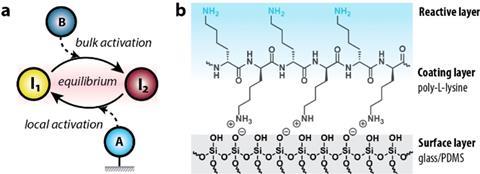
The equilibrium system consisted of a simple acid-base reaction, i.e. protonation and deprotonation. Initially, the researchers saw that a single layer of polylysine made deprotonation slightly slower than protonation, but the effect wasn’t as strong as expected. ‘It took us a long time to understand why that was’, says Wong. ‘We started with one layer to keep it simple, but according to a mathematical model we should have seen a stronger effect.’
The solution turned out to be making multiple layers of polylysine. Wong: ‘This was surprising in two ways: on the one hand, the effect of three layers instead of one was much stronger than expected; on the other hand, the difference between three layers and nine or more layers was negligible.’ These so-called polyelectrolyte multilayers made deprotonation much faster, which significantly slowed down the equilibrium. Wong: ‘I’m thankful we got to work towards this solution as a team. Our synergy turned out to be highly effective.’
‘In a general sense, this should work for many types of equilibria’, Wong continues. ‘With these surface-bound reactions, you can affect equilibria in a very different way than you can in a beaker.’ And that could be interesting for applications, because you can influence feedback loops very directly and understand what makes that feedback possible in the first place. ‘In chemistry, you usually measure in time, but surface reactions add a spatial component that allows you to detect dynamically, which can lead to biosensor applications.’
Wong’s group is taking this concept further. ‘We kept it simple with channels from the 3D printer, but now we want to create spatial gradients on a smaller scale: make smaller channels, create gradients in them and then look at the effect on sensitivity. He also wants to move towards a more complex inner layer.’ At the moment, these are rather simple amine groups, but the group also sees opportunities in DNA and thiol chemistry. ‘This functionalisation with more biochemical components makes it more relevant for studying feedback loops in life.’
Koyuncu, A.H. et al. (2023) ChemSystemsChem e202300030, DOI: 10.1002/syst.202300030












Nog geen opmerkingen