Research into plastic recycling with sulphated zirconium oxide took an interesting fundamental turn when a team of Dutch and American chemists found signs of transient superacidity, as reported in Angewandte Chemie International Edition.
‘Our group has been collaborating with the group of Matthew Conley from the University of California, Riverside for years’, says Evgeny Pidko, Professor of Inorganic Systems Engineering at the TU Delft. ‘They shared a very unusual case with us about some of their organometallic surface chemistry experiments with zirconium.’
Conley and his colleagues were working on breaking down polypropylene and polyethylene into smaller pieces with sulphated zirconium oxides (SZO). ‘They got small hydrocarbons, which could find a use in aviation fuels’, says Pidko. ‘However, they didn’t quite understand how the C-H bonds were activated .’
Inspiring
Upon further investigation, the ‘usual suspects’ for this kind of activation, i.e. the metal sites and the sulphuric acid, were ruled out. ‘Earlier research points to σ-bond metathesis, heterolytic cleavage mechanisms and Brønsted sites as the cause for the activation, but that’s not what we observed’, Pidko explains. ‘We calculated all the different mechanisms and none of them could explain this reactivity. The sites that are readily available for the adsorption and interaction on the equilibrium surface show moderate reactivity, which is not enough to cleave the bonds. However, under the reaction conditions, something happened to facilitate it.’
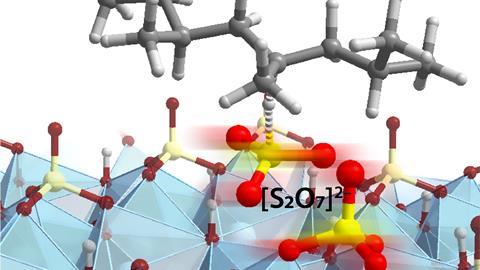
One inspiring evening – ‘We meet with Matthew’s group at 9 PM because of the time difference’ – and some DFT calculations later, Pidko and his postdoc Alexander Kolganov discovered that the adsorbed pyrosulphate can briefly open up to an adsorbed SO3 that acts as a superacid and is stabilised by the reactant. ‘It starts reacting with the polymer immediately, and afterwards disappears again. It’s something we wouldn’t have thought about without the calculations.’
Eye-opening
Pidko found it eye-opening to see that a non-metallic reactive ensemble could act as a catalyst on the surface of the metal oxide . ‘It shows that catalytic reactions can happen at transient reactive sites, something that really challenges the catalysis field.’ He explains: ‘The main goal of catalyst design is to connect the thermodynamic properties of a material with the kinetics of a catalytic reaction. In other words, you want to connect the low “valleys” of the ground states defining the material on the shelve with the reaction barriers it is going to catalyze. However, it turns out that these barriers are rather defined by the transient species and their properties, which have a very elusive nature and emerge through the synergy between the reactant and surface ensembles right before the reaction takes place.’
Opportunities
A logical next step for Pidko’s group is to computationally explore the reactivity of similar acids like boronic and phosphoric acid. ‘We want to understand how you could modify this heterogeneous catalyst and make the most use out of the transient sites that pop up.’
The discovery gives rise to opportunities for new catalyst design approaches, especially when looking at opening other anhydrides and seeing how the reactivity changes. ‘As a catalysis person, we constantly look for new tools to create reactive assemblies. If you can coat a surface with inert molecules which open under particular conditions, you have a lot more control over the kinetics of the product, due to the transient nature of the active sites.’
Plan
A peculiar detail about this study is that it was not a result of any project. ‘This research was not supported by any institution’, says Pidko. ‘We relied on the supercomputer infrastructure that was available for the calculations.’ To him, the project shows that you can’t plan discoveries or innovations. ‘I’m convinced that it’s not the funded projects that land the discovery, but that it happens along the way when you least expect it. In my experience, the most beautiful findings don’t come from the projects you apply for.’
Jammee, R. et al. (2024) Ang. Chem. Int. Ed. e202421699, DOI: 10.1002/anie.202421699












Nog geen opmerkingen