Attaching a biotin tail to a cancer drug makes it possible to see very specifically which proteins the drug targets in lysed cancer cells, as a Dutch team shows in ChemBioChem.
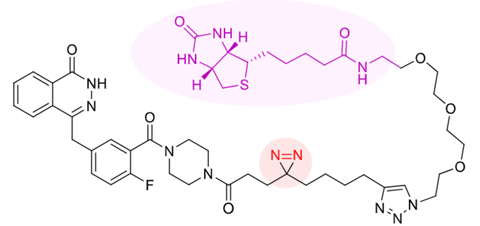
Olaparib is a commonly used drug for breast and ovarian cancer. It is known to inhibit the PARP1 and PARP2 enzymes, but any off-target activity could cause unwanted side effects. To get a complete picture of what else the drug binds to in the cell, researchers from Leiden University, the NKI and Radboud University decided to give the drug a ‘biotin tail’ that can be pulled out later when the drug has bound to other proteins or enzymes.
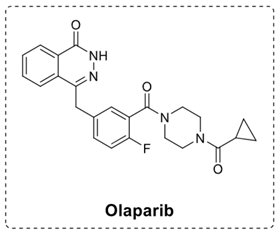
Something similar has already been done: you give the drug an alkyne tail that you can attach to an azide using copper-click chemistry. The azide is attached to a fluorophore or biotin, which you can use to detect the capture by imaging or mass spectrometry, respectively. The only disadvantage of this copper-click chemistry is that it can be harmful to the cell in which it is used, and therefore disrupt the potential targets.
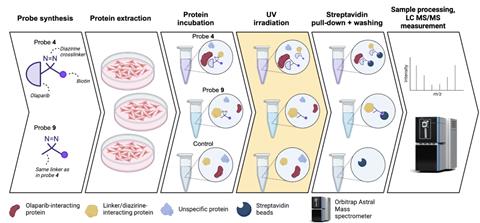
To get around this, the researchers decided to attach the biotin tail to the drug in advance. They also added a photocrosslinker to olaparib, in this case a three-membered ring with an N=N section. The drug itself binds to targets, but this is often reversible. With the crosslinker, you can – after exposing it to UV light – permanently attach olaparib to any target.
But how can you be sure that the targets you find are actually bound to olaparib and not to the biotin tail? The team thought of that too: simply take the biotin tail without the drug and compare the signals with those from olaparib. The researchers found that olaparib binds not only to PARP1 and PARP2, but also to the kinases CDK8 and CDK19.
They hope that this method could be a tool to help reduce off-target effects of new drugs.
Van der Heijden, F.L.A.M. et al. (2025) ChemBioChem e202400882, DOI: 10.1002/cbic.202400882












Nog geen opmerkingen