In Nature Chemistry, researchers from the University of Groningen present a minimal system of two self-replicating ring structures capable of Darwinian evolution.
Step by step, the group of Sijbren Otto, professor of systems chemistry at the University of Groningen, adds another property or pathway to their molecular system that should eventually - albeit at a very basic level - start behaving like a living entity.
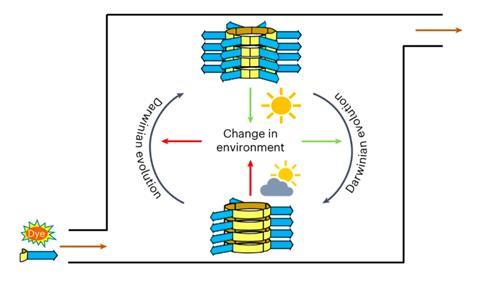
Their latest addition: eco-evolutionary dynamics. Put differently; an interaction between molecules and environment that allows Darwinian evolution. Survival of the fittest in a fume hood. It all started, more than eight years ago, with the discovery of so-called replicators: self-assembling macrocyclic structures, based on a benzene 1,3-dithiol with an amino acid tail, which stack into tiny fibres. Soon after, they demonstrated that these replicators are capable of species formation, another Darwinian concept: different ring sizes are formed, based on the same building block.
Subsequently, these replicators were also found to be able to accelerate their own formation or, after a slight modification in the building blocks, to engage in photocatalysis to replicate even faster. Moreover, in the latter case, it was found that the replication process, by producing singlet oxygen, also changed the oxidation state of the environment.
Opposite effect
All these previous steps now come together in a flow reactor system in which, starting with the usual building block, two different replicators emerge: rings composed of three building blocks, here termed R3, and rings composed of six building blocks, R6. Under the influence of light, the replicators change the oxidation state of the environment, and this changed state in turn affects the replication rate - the ‘fitness’ - of the replicators. And, there’s the crux, that altered oxidation state has an opposite effect on the two replicators.
‘A high oxidation state, due to the production of singlet oxygen, is problematic for the six-rings,’ Otto explains. ‘These can no longer replicate fast enough in the flow reactor and therefore “die”.’ In this situation, R3 gets the upper hand. If the oxidation state drops again, the situation alters. ‘At a low oxidation state, it becomes too difficult for R3 and R6 wins.’
However, evolution involves more than just an interaction between ‘organism’ and environment. There must also be selection: a certain trait offers an advantage/disadvantage and will therefore increase/decrease within the population. Otto: ‘The evolutionary dynamics lie in the fact that sometimes mutations occur and we define them as an error during the copying process. When, for example, R6 does not produce another R6, but instead an R3. Or vice versa. It’s still a copy, but it’s ’s the wrong one. That happens in our system. Not often, just as it doesn’t happen often in biology, but when it does the mutation propagates. If R6 arises from a fault in R3 replication, it will replicate further as an R6. The selection is fuelled by the changing oxidation state of the environment; if it is favourable to the mutant, it will replicate faster and thus the “fitness” of that mutant increases.’
Mechanistic explanation
In the end, most of the work wasn’t in getting the system up and running, but in explaining what was actually happening, Otto says. ‘It took a long time to figure out the mechanistic explanation. The behaviour was absolutely clear, but it was still a mystery why sometimes R6 replication led to an R3, and why at high oxidation states R3 performed best.’
Ultimately, the explanation was in the mechanism of replication. ‘Normally, replication proceeds via sulphide chemistry, when a thiolate causes the disfulfides to break, which allows new rings to be formed. But at a high oxidation state, there is no thiolate present and that replication route becomes impossible. However, with R3, a radical-based mechanism still functions. That is not very effective, but because the other mechanism no longer occurs, the radical mechanism “wins” and R3 can still replicate.’
Generic descriptions
Meanwhile, the next publication has already been submitted to Nature Chemistry, as the team has still plenty to work out. ‘We have now shown that eco-evolutionary dynamics occur in the most minimal system, but that is also its limitation. We want to achieve a higher degree of evolvability; bringing multiple layers into the system that enlarge the possibilities for variation thus evolutionary development,’ Otto explains. ‘In this new paper, we use two different building blocks and show that this sometimes leads coexistence and sometimes to competitive exclusion, where only one species remains. Again, the mechanistic explanation was the biggest hurdle to scale.’
Mutation, selection, evolution, propagation, fitness: applying these well known, but also often misinterpreted concepts, to a non-biological system means venturing into quite a minefield of interpretations and definition issues. But that didn’t cause much discussion, says Otto. ‘As long as you clearly define what you mean. It didn’t pose a problem for the reviewers.’ Moreover, he says, it is essential to opt for a broader interpretation of these concepts. ‘The context of biology established the terminology, because that is the only example of biochemistry and life that we know. But these phenomena are relevant on a broader scale and we therefore want to describe and use them in a more generic manner.’












Nog geen opmerkingen