Ben Feringa’s group has developed a simple, green method for attaching unprotected alcohols to amino acids, with only water as a by-product. All the possibilities are described in Chemistry A European Journal.
The Groningen researchers and their American and Chinese colleagues follow the principles of green chemistry, focusing on atom economy, low waste and the environmental factor (mass of waste divided by mass of product). They are combining these principles with known reactions: creating surfactants based on amino acids and creating C-N bonds by ‘borrowing’ hydrogen. This allows them to attach unprotected alcohols to amino acids in a single step.
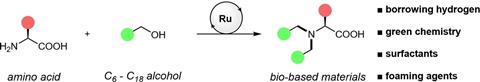
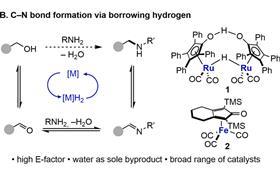
This borrowing occurs via a commercially available catalyst - in this case based on ruthenium - which captures a hydrogen atom from the alcohol and oxidises it to an aldehyde. Next, an imine is formed with the amine group of the amino acid, which is then reduced to an amine.
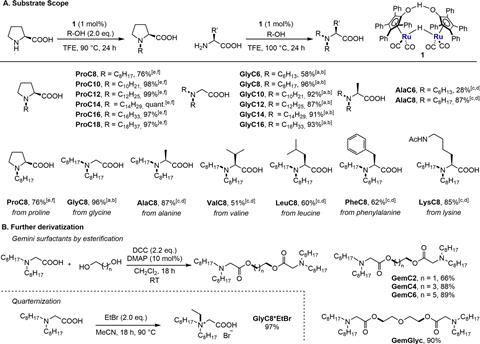
After optimising this principle for proline, glycine, alanine and octanol, they varied the length of the alcohols and allowed them to react with four other amino acids. In this way, they also synthesised a Gemini surfactant, a combination of two surfactants linked at the head, and a quaternary ammonium salt.
They then carried out tests on the foamability, biodegradability and toxicity of the surfactants and found two that they considered promising: alanine coupled to octanol (AlaC8) and lysine coupled to octanol (LysC8). The researchers conclude that both are readily biodegradable, non-toxic and produce good, long-lasting foam. They are therefore candidates for further development. The Groningen researchers will now investigate whether catalysis is also possible with first row transition metals.
Koy, M. et al. (2025) Chem. Eur. J. e202500077, DOI: 10.1002/chem.202500077












Nog geen opmerkingen