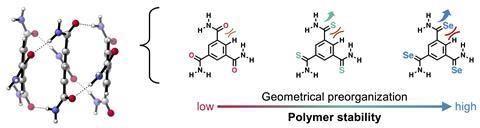
Hydrogen bonds are widely used in biosupramolecular systems and their synthetic equivalents. This also applies to hydrogen-bonded polymers. Recent research has shown that replacing the oxygen atom in benzene-1,3,5-tricarboxamides (BTAs) with sulphur or selenium produces a more stable polymer. The reasons for this were not very clear, but Celine Nieuwland, Célia Fonseca Guerra and colleagues at the Vrije Universiteit Amsterdam have come up with a disarmingly simple explanation.
’It all started last year’, says Nieuwland. ’Célia and I published a paper on a question in organic catalysis: experiments showed that thioamides [amides with sulphur, ed.] are better hydrogen bond donors than “normal” amides with oxygen.’ The scientific community proposed resonance structures as an explanation, since one would expect the opposite based on electronegativity, but the Amsterdam team was not satisfied with this explanation.
’What we found was that it had to do with the size of the atom, more precisely with the steric Pauli repulsion’, explains the PhD student. The larger the atom X in the amide C=X bond, the stronger the steric repulsion and the longer the C=X bond. This bond extension lowers the π*C=X orbital, which ‘pulls’ electrons away from the nitrogen atom. ’This makes the amino group slightly more positively charged and therefore a stronger hydrogen bond donor.’
Obvious
The researchers saw a similar effect in the supramolecular chemistry of the BTAs. ’We wanted to know how our fundamental finding on amides could be applied to supramolecular chemistry’, Nieuwland says. ’The explanation from the field was again based on resonance structures, but this does not correspond to reality. So we investigated how it really works.’
There are several reasons why sulphur BTAs and selenium BTAs give a more stable polymer. Because sulphur and selenium are larger than oxygen, there is more disruption in the structure of the monomers. This makes it easier for the monomers to bond together. Nieuwland: ’It was quite obvious, but the experimenters missed it. It is an important point for material design though: you have to look closely at the structure of your monomers in a polymer, because small variations can make a big difference, for example in the formation of hydrogen bonds.’
Haemoglobin
A second reason has to do with last year’s findings. Amides with sulphur or selenium give stronger orbital interactions and therefore stronger covalent interactions in the hydrogen bonds,’ explains Nieuwland. ’At the same time, a larger atom has more electrons, so you have more and better dispersion or Vanderwaals interactions.’
The covalent interactions in the hydrogen bonds also ensure that adding a monomer to a BTA dimer is more stable than forming a dimer. ’You may compare it to haemoglobin. When haemoglobin binds one oxygen molecule, the affinity to bind another oxygen molecule increases. It works the same way in these polymers concerning the strength of the hydrogen bonds.’ Nieuwland concludes: ‘I think the paper shows that quantum chemical calculations are really important for understanding macromolecular structures, not just small molecules.’
Nieuwland, C. et al. (2023) Chem. Eur. J. e202300850, DOI: 10.1002/chem.202300850












Nog geen opmerkingen