Researchers in Ghent have found a method to determine the temperature of a catalyst very locally during a reaction, as reported in Nature Catalysis. Their work offers a new fundamental view of catalytic reactions.
‘The vast majority of the literature on catalysis deals with the relationship between the structure of the catalyst and its performance’, states Matthias Filez, assistant professor at Ghent University. This is not surprising, of course, since the structure influences which molecules can bind to it. ‘But what is often overlooked is that the temperature of the catalyst can change due to chemical reactions. The laws of thermodynamics and kinetics tell us that this temperature is an important parameter in determining how fast reactions can take place and what products can be formed. If the temperature of the catalyst changes, the performance potential can also change.’

A handful
Only a handful of publications have looked at measuring the local temperature on the catalyst, for example by researchers in the Weckhuysen group at Utrecht University. ‘This is probably due to the difficulty of characterisation’, explains Filez. There were no good methods for determining the temperature at the exact location where the reaction takes place. Once we realised that, we turned the proverbial lens on it, and our advanced X-ray technique came in handy.’
Filez is referring to EXAFS thermometry, which allows you to take a kind of X-ray photograph, not of bones, but of nanoparticles – i.e. the catalytic component that carries out the reaction. You can use it to look at the structure, but our trick was that the structure of a particle is also affected by temperature.’ As the temperature rises, so does the disorder in a system, which can be measured using X-rays produced by a large synchrotron (see image above). ‘We then linked the disorder to the temperature. Researchers generally consider the effect of temperature in EXAFS to be an obstacle because it affects the signal. But we turned this disadvantage into an advantage.’
Special publication
For Filez, this publication is a special moment of coming full circle. ‘The co-authors are senior colleagues at Flemish universities who have supported me throughout my scientific career. Professor Christophe Detavernier, for example, was my co-supervisor during my PhD and an authority in the field of X-ray methods. Professor Jolien Dendooven is also an important sounding board for me, and in this way all the co-authors have been very important in the realisation of this publication and my own development as a scientist.’
Besides ‘fundamental curiosity’, Filez and his colleagues also recognise the practical importance of this research. Take a car, for example. It has all kinds of parts, but when it doesn’t run, you look first at the engine, which is the core of the propulsion system.’ You can look at the catalyst in the same way, says Filez. ‘The nanoparticles on the substrate form the engine of a catalyst. So if you want to know why a catalyst works or not, you have to look locally at the nanoparticles.’
A surprise
The analysis of the literature brought the team a surprise. Filez: ‘In collaboration with the Chemical Technology Laboratory at Ghent University, we started analysing data sets. ’The result was astonishing: we saw local temperature changes of 90 to 100 degrees Celsius! Our biggest challenge was to convince ourselves that our analysis was correct.’
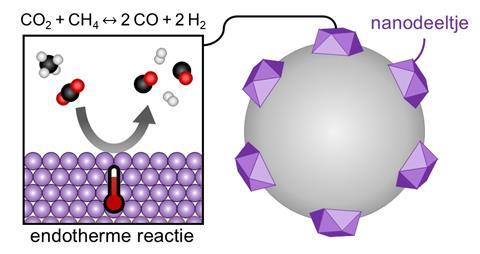
So they dug even deeper into the data and discovered a consistent line. ‘The temperature really does vary, sometimes by tens of degrees. We then started to test this ourselves with the methane reforming reaction. This reaction is endothermic and therefore needs heat from the catalyst to continue.’ It also turned out that the nanoparticles acted as a kind of energy sink, causing the big drop in temperature. The next question was: does this only apply to endothermic reactions, or do you see a reverse phenomenon with exothermic variants? The latter appeared to be the case, although the effect was less pronounced.
Tipping point
‘That was really the tipping point for us, it was confirmation that our method worked’, Filez continues. ‘We then developed advanced methods to quantify exactly what these temperature changes were.’ According to Filez, the work shows that the current literature gives an incomplete picture of catalysis. ‘The aim is still to establish structure-function relationships, but in fact you need to establish structure-temperature-function relationships, because temperature plays an important role in the reaction rate, for example. This changes our fundamental understanding of the subject.’
What is certain now is that the temperature of nanoparticles changes. Filez: ‘So the first fundamental aspect we want to work on is to establish structure-temperature-performance relationships. We also want to find out what the underlying mechanisms are in the energy management of the catalyst itself. The temperature measured is a macroscopic property involving many energy flows.’
Finally, this discovery opens the way to the development of a new generation of catalysts. ‘We want to develop tools that allow us to control the temperature of the nanoparticles, so that you can have more stable processes in the chemical industry.’
Filez, M. et al. (2025) Nat. Catal., DOI: 10.1038/s41929-025-01295-9













Nog geen opmerkingen