A team from Amsterdam has 3D printed a catalyst made entirely of stainless steel and aluminium that works extremely well for borohydride hydrolysis. Unexpectedly, it performs even better than a previous version made with cobalt, they report in ChemSusChem.
The field of catalysis is constantly looking for catalysts that do not contain expensive and rare metals, for example for the storage and release of (green) hydrogen. While trying to improve a specific cobalt catalyst, Frances Pope, Gadi Rothenberg and colleagues at the University of Amsterdam discovered by chance that the base material for their catalyst actually works even better without cobalt.
They made this base material by 3D printing a mixture of iron and aluminium powder using selective laser melting (SLM) and then treating it with acid and base. Typically, they would then load the resulting catalytic reactor monoliths with cobalt, but in a control experiment without the metal, they were surprised to find that this variant was also very active and stable, both in batch and flow systems.
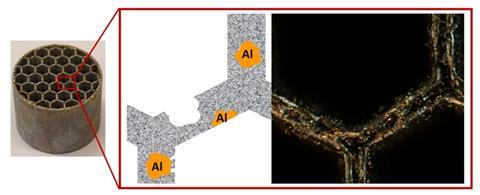
The purpose of the catalyst in this case is to release hydrogen from potassium borohydrides, solid crystals that can act as hydrogen carriers. The fact that you don’t need cobalt for this is a nice bonus, but an added advantage is that aluminium and stainless steel are both easy to recycle and relatively cheap: just fifty cents per monolith. The fact that you can also 3D print means that there is almost no material left over. What is left over can be reused.
The researchers hope that this discovery will encourage more groups to try these methods, as cheap materials, a simple production method and high chemical activity open up a world of catalytic possibilities.
Pope, F. et al. (2024) ChemSusChem e202401264, DOI: 10.1002/cssc.202401264












Nog geen opmerkingen