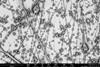
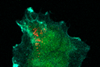
Serendipity is a common thread running through many extraordinary discoveries in the history of chemistry. This image of ‘molecular jellyfish’ is no exception. ’It is completely useless, but incredibly beautiful.’
Tieze van den Elsen, who is currently finishing his Master’s internship at Radboud University, makes polymers in the molecular nanotechnology group of Roeland Nolte and Hans Elemans. Specifically, he is working with helical polyisocyanides. We try to control this helix shape, i.e. whether it turns to the right or to the left, with the molecular motors of the Feringa group,’ says Hans Elemans, associate professor and Van den Elsen’s supervisor. Van den Elsen started his internship in November and produced his first polymer in December.
The last step in polymer synthesis is purification,” Elemans continues. You then precipitate your substance in a liquid in which the polymer does not dissolve. But something strange happened during Van den Elsen’s purification: ’Tieze sent me a picture of the precipitate that had formed; I had never seen anything like it. It reminded us of molecular jellyfish.
The two explained it to Nolte, who understood what had happened. Elemans: ’Normally you have to stir well when precipitating to get a powder, but Tieze had not stirred it, and then apparently you get these jellyfish-like shapes. The shapes themselves are completely useless and we still don’t understand exactly what happened, but it shows again how incredibly beautiful chemistry can be.












Nog geen opmerkingen