A 2023 paper in JACS suggested that the definition of hydrogen bonding should be extended to include hydrides. A team from Amsterdam recently responded in the same journal to argue for keeping the hydrogen bond definition as it is.
The International Union of Pure and Applied Chemistry (IUPAC) regulates the naming and definitions of chemical terms. It is vital that these definitions are clear; a wrong definition can lead to wrong theory and ultimately wrong applications.
Take the hydrogen bond (H-bond). IUPAC defines it as ‘an attractive interaction between a hydrogen atom from a molecule or a molecular fragment Y–H, in which Y is more electronegative than H, and an atom or group of atoms (Z), in which there is evidence of bond formation (i.e., a Y–H···Z hydrogen-bonded complex)’. This bonding motif is very well defined and understood: the bonding mechanism is a HOMO–LUMO interaction between the lone pair of Z and the σ* acceptor orbital of Y–H. This mechanism forms the basis of much theoretical and practical knowledge.
A worrying proposal
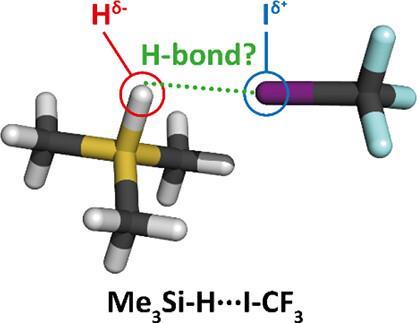
However, in a paper published in JACS in 2023, a team of Czech researchers led by Svatopluk Civiš and Pavel Hobza suggested that the IUPAC definition was incomplete. ‘We found it quite worrying’, say postdoc Lucas de Azevedo Santos and professor Célia Fonseca Guerra from the TheoCheM group at the VU Amsterdam. ‘Their proposal was suggesting that what we knew about hydrogen-bonding was incorrect. It would completely change the theory and concept behind hydrogen bonds.’
In their paper, Civiš, Hobza and colleagues added just two words to the IUPAC definition (emphasis mine, ed.): ‘The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more or less electronegative than H and an atom or a group of atoms in the same or a different molecule in which there is evidence of bond formation. A typical hydrogen bond may be depicted as X–H···Y–Z, where the three dots denote the bond.’
‘Essentially, they say that a negatively charged hydrogen atom can act as a hydrogen-bond donor’, Fonseca Guerra explains. ‘They suggest that hydridic hydrogens in X–Hδ– can accept charge from a lone pair on Z. But then you’re talking about donating electrons to an already negatively charged hydrogen atom with a very high energy σ* LUMO, and our knowledge on hydrogen bonding would say that’s not favourable.’
Healthy debate
Together with VU colleagues Pascal Vermeeren and Matthias Bickelhaupt, they decided to write down what they believe an H-bond is and is not, and publish it in the same journal. ‘This way we reach the same audience, so they can see both sides of the argument’, adds de Azevedo Santos. ‘It makes for a healthier debate.’
The Amsterdam team came up with some simple arguments backed up by computational chemistry. ‘First, we took ammonia, because it is a known fact that ammonia can act as hydrogen-bond acceptor’, the postdoc continues. ‘We then modelled a set of monomers with negatively charged hydrogen atoms – the hydrides – and a set of monomers with positively charged hydrogen atoms – the protons. The hydrides do not form hydrogen bonds with ammonia whereas the protonic hydrogens do, in line with the traditional IUPAC definition.’
On the nature of bonds
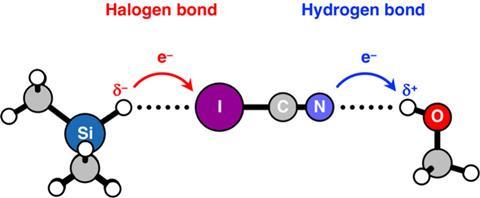
If protons form H-bonds, what kind of bonds do hydrides form? This was the next question the team set out to answer. ‘We deliberately chose our second system to be I-C≡N’, says Fonseca Guerra, ‘because it can form hydrogen bonds on the N side and halogen bonds on the I side.’ As a source of H atoms, they used MemY-H molecules, where Y can be C, Si, Ge, N, P, As, O, S or Se. Depending on the Y atom, it would be more electronegative or electropositive than the H atom.
‘Firstly, we confirmed that hydrides can only bind to the iodine side. Next, we looked at the charge donation from the lone pair on the I atom to the σ* LUMO of the hydrides and found that it’s very weak’, says de Azevedo Santos. ‘The point is that hydrides are bond acceptors, not donors – they donate charge to a different monomer. The bond between a hydride and the iodine atom in ICN would be classified as a halogen bond. If you go through all the different atoms in Y in MemY-H that give rise to hydrides, the hydride is always a bond acceptor, not a bond donor.’ Fonseca Guerra adds: ‘Indeed, and we see that the hydrides always form halogen bonds to iodine in I–CN, and donate charge into the σ*I–CN, whereas the protonic hydrogen always binds to nitrogen in NC–I and accept charge from the lone pair on nitrogen.’ The conclusion: there’s no need to change the IUPAC definition.
The redshift argument
‘The focus of Civiš and Hobza’s argument was based on spectroscopic criteria, the so-called redshift’, de Azevedo Santos continues. When a hydrogen bond is formed, the lengthening of the Y–H bond can be observed in the form of a redshift in the IR spectra. Something similar happens in a hydridic complex. ‘But the observation does not support the conclusion that they are also hydrogen bonds.’
What then is the reason for the redshift? ‘In hydrogen-bonding, it is well known that the Y–H bond stretches when accepting electrons in its antibonding σ*Y–H orbital’, explains de Azevedo Santos. ‘What we have shown is that, in the hydridic situation, the Y–H bond stretching is due to its bonding σY–H orbital losing electron density. This means that there are two different reasons for why bond-stretching occurs.’
Right or wrong?
In their JACS paper, the Amsterdam team writes: ‘We falsify the conclusion that interactions involving protonic and hydridic hydrogens are both hydrogen bonds; they are not.’
For Fonseca Guerra, it’s not so much about pointing out that Civiš and Hobza are wrong. ‘I want it to be more constructive. How can we construct our arguments to convince our readers, so that they can decide for themselves which idea is right or wrong? It’s not us against other researchers but taking the reader through the manuscript so they can see how our arguments are built up.’
‘Scientific debate is, at times, sensitive and provocative’, says de Azevedo Santos. ‘But it’s an interesting experience, it makes you think and go back to understand how things work.’ He hopes that this debate will be accessible to the general public. ‘These fundamentals are important to understand. It shows that we all need to be careful about drawing conclusions from observations. Sometimes there’s more to it than meets the eye.’













Nog geen opmerkingen