For perfect oligomers, you need to look beyond synthesis. Assembly conditions are also essential, says Chemistry A European Journal.
What do Marvel’s Avengers and oligomers have in common? Just assembling them in any given way is not the best approach. You need a plan. How you assemble oligomers and what the processing conditions are, is just as important as your design of the molecular structures. ‘The precision with which you can synthesize oligomers plays a big part in why they’re so popular in the field of nanotechnology’, says Ghislaine Vantomme, Assistant Professor Macro-Organic Chemistry at TU Eindhoven. ‘In nanotechnology, you need to prepare structures of high precision that are perfectly defined at the molecular level, reducing domain spacings and improving long-range order, so you can steer the function of the materials you want to make.’

Biological precision
Discrete oligomers, Vantomme says, are better suited than polymers, since the length of the latter varies, whereas the former will always have the same length when you synthesise them. One of her research goals is to push the synthesis of these molecularly perfect oligomers to get interesting morphologies and unusual properties.
An example of this is found in a recent paper published in Chemistry A European Journal, which was a collaboration between Vantomme, Bert Meijer (TU Eindhoven), Steven De Feyter (KU Leuven) and Alain Jonas (UC Louvain). The groups worked together on naphtalenediimide (NDI) oligomers that self-assemble. ‘It is part of a bigger FWO and FNRS funded Excellence of Science project which looks at sequence-defined polymers to engineer materials with biological precision’, says Vantomme. ‘A prevalent idea in the field currently is that if you’re just very precise in the synthesis of the oligomer, you’re done. Unfortunately, it’s much more complicated than that.’
‘It was hard to synthesise a decent amount’
Challenging
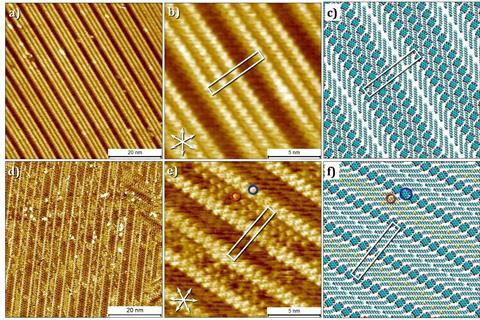
The paper covers the synthesis and characterisation of the NDI oligomers in bulk, in solution and at the liquid-solid interface. Vantomme: ‘We synthesised the molecules at Eindhoven, Jonas helped to characterise the structures in bulk and Steven and his group used their expertise in scanning-tunnelling microscopy to characterize the interface.’ The synthesis was especially challenging, taking first author Christiaan Corbet and Bas de Waal a very long time to get their hands on the oligomers. ‘It required many steps, the solubility was bad, and it was difficult to ensure the purity of the compound, which made it hard to synthesise a decent amount.’
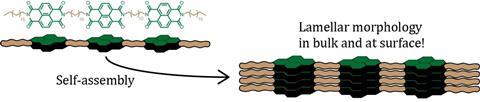
That wasn’t the only challenge. ‘The oligomers form nanostructures. But when we characterised them, we were a bit disappointed at their ordering’, Vantomme says cautiously. ‘Due to the length of the unsaturated chains that hold the NDI together, the conformational flexibility makes the nanostructures less than perfect. But we couldn’t do without the long chains because of solubility.’ Despite the challenges, this demonstrated the major idea of the paper. ‘It showed how important it is to control the pathway selection and the processing conditions to get the nanostructures you want.’
Applications
And when you do that, it is possible to get some pretty neat structures. To cite the paper: ‘In general, the NDI oligomers tend to form lamellar morphologies via crystallization-driven assembly. The oligomers with shorter linkers preferably assemble in an extended conformation, while the oligomers with longer linkers show a mixture of folded-chain conformations and disordered morphologies due to the presence of the unsaturated spacers.’
What kind of applications does Vantomme have in mind? ‘My dream is to use supramolecular materials for brain-inspired computing’, she says, to which she adds laughing: ‘Maybe this material could be used for a new superpower of the Avengers, but we’re not there yet.’ Vantomme notes that this research is also more on the fundamental side. ‘Besides, the difficulty in synthesis hampers applications too.’
Nonetheless, Vantomme thinks this is important work for the nanotechnology field and she appreciates the collaboration. ‘Collabs are essential to further the field, because it becomes so complex when you combine interdisciplinary fields, synthesis and analysis. I’m very pleased that we can cross borders with this type of funding.’ To conclude with the authors: ‘With this study the frontiers in oligomer synthesis, molecular analysis, and ordered supramolecular assemblies of precise oligomer materials are pushed forward, while at the same time the limitations in this endeavour are made clear.’
Corbet, C.H.W.A. et al. (2023) Chem. Eur. J. e202303107, DOI: 10.1002/chem.202303107 (Open Access)












Nog geen opmerkingen