Making molecules with bacteria and electrochemistry, that’s what drives Ludovic Jourdin in a nutshell. This relatively new field is developing quickly and has much to offer. ‘We work multidisciplinarily from the nano scale up to the industrial scale.’
Ludovic Jourdin, Associate Professor of Biotechnology at Delft University of Technology, is usually very enthusiastic, he says while we take the stairs to a quiet spot on the second floor of the Department of Biotechnological Engineering. ‘But it’s been a very busy week and a thunderstorm kept me awake last night. I’ll try to be as enthusiastic as normal, though.’ And he kept his word; with great animation and his smile never far away, Jourdin shares his work in microbial electrosynthesis, a relatively new field of which he was one of the pioneers.
You have seen quite a few places around the world: occupational trainees in Canada, France and New Zealand, in addition to a PhD in Australia. What is the most valuable lesson you learned during those years?
‘It’s been a very enriching journey for sure. On the one hand I got to experience many different cultures, on the other I got to see many different labs and how they operate. It gave me the tools to build my own group here at Delft. That’s why I always advice my students to gain experience in many different workplaces, ideally in several countries. It opens up your eyes like crazy.
I come from a small village in France and I couldn’t speak a word of English. But to further my career, I had to have an English diploma. So I took a risk and went on to do an internship in Canada – the English-speaking part – for a year. That’s where it all started for me.’ [laughing]: ‘I remember being on the plane and thinking to myself, “what am I doing?”. It was a tough start, but it really opened up my eyes.’
Five years ago, you settled for the Netherlands. What brought you here?
Jourdin shows a big smile: ‘That was a bit of an unexpected direction. During my PhD, I was part of one of the first groups that began working on microbial electrosynthesis. When I started, there were no papers written about the subject. I became pretty successful and had a great life in Australia, but obtaining funding for research that is not mainstream is very difficult there. Because of that, I’ve seen many great PIs rise and fall. So I thought, I’m European, let’s go back to Europe.
‘With bacteria we can create more complex molecules or even food’
In 2014, I attended a few conferences in Switzerland and Spain, and people from Wageningen University noticed me and invited me to the Netherlands and put a contract in front of me. I was in my final year and had not started looking at job applications, so when I was offered that postdoc position, I thought, why not! After a few years at Wageningen, a friend and former classmate of mine was doing a PhD at Delft and saw a vacancy, which she thought would fit me well. But the application deadline had already passed. I applied anyway, was invited and got the job.’
For good reasons, I suppose.
‘Yes, TU Delft is a great university and innovation hub. But I did my homework too and I was attracted by the department of biotechnology, thinking if I was to set up a group on microbial electrosynthesis, surely this was the place to be. I knew the PIs here could help me develop this technology further. I’m not an individualist, I hate working alone, and it’s impossible to know everything about this subject. The technology I’m working on is very multidisciplinary, so you really need as much expertise as you can find.
Another reason I accepted was that the e-refinery institute was being set up. Initially, it was a TU Delft-wide initiative with about fifty PIs. The leading thought was that electrochemistry is the way to go for the industry. Knowing this, I thought it would be a perfect mix: to be in a great department for biotechnology and have people around me that I could benefit from in terms of the electrochemistry perspective was really a no-brainer.
Another part that really excites me is supervising students from all levels. Seeing them grow and making an active contribution to the field, that’s the best part of my job. I get a lot of satisfaction from teaching them, brainstorming with them, getting new crazy ideas to try. Initially, I took this job for the research, but during my time here I’ve really come to enjoy teaching and I believe we can make a great impact this way.’
Article continues after image

What gets you excited about microbial electrosynthesis?
‘Our society is based on carbon that comes from fossil resources. We all know the bigger picture: we need to get rid of fossil resources and create more sustainable products, and what better way to do this than to use CO2 and renewable energy? But there’s more to it for me. I’m an application-oriented guy, I want to make an impact on society, but at the same time, I really want to understand what I’m doing. In my view, if you want to apply a technology, you need to fully understand it. So, we do both fundamental and applied science, which I think is one of our strengths. Working with bacteria adds a layer of complexity, but it also creates a clear distinction from other technologies that are being developed with similar feedstocks.’
What is the advantage of microbial electrosynthesis (MES) when compared to ‘normal’ electrosynthesis?
‘When you talk about CO2 conversion in normal electrosynthesis, you see very high conversion rates for small C1 and C2 molecules, methanol, ethylene, CO, to name a few. But a challenge for those systems is stability. After a couple of hours or days, you usually lose performance. The advantage of microbial electrosynthesis is that we can continuously operate reactors for three years without any major issues. And bacteria are robust. If a small problem occurs, like a leaking pump, and you fix it within a relatively short time, the bacteria come back to life and are happy again.
‘We work from the nanometre scale all the way up to industrial scale’
Take CO2 electrolysers for example. They use exactly the same feedstock but instead of bacteria, they use heterogeneous catalysts. Now, I’m not saying it should be one or the other, both should be developed simultaneously. Electrolysers are better at making products with one or two carbon atoms, but with bacteria we can create more complex molecules with more carbons or even food from CO2 and electricity.
Both technologies are not mutually exclusive, they don’t compete. We target different markets and applications. We can make more complex and valuable products with MES, which means we don’t have to have the same high rates. A final advantage is that we use cheap carbon-based materials for the electrodes and bacteria, which are basically free, while classic electrocatalysis is based on metals, for which challenges may arise in the long run.’
But there are challenges for MES too, I reckon.
[Laughs] ‘Sure, I would not have a research group if there were none. We always ask ourselves: what is limiting MES and what can we do to overcome those limitations? We use a multidisciplinary and a multiscale approach, from electrode surface chemistry to microbes and biofilms, up to reactor scales, stacks of electrochemical systems and finally system integration at the industrial scale.
Article continues after image
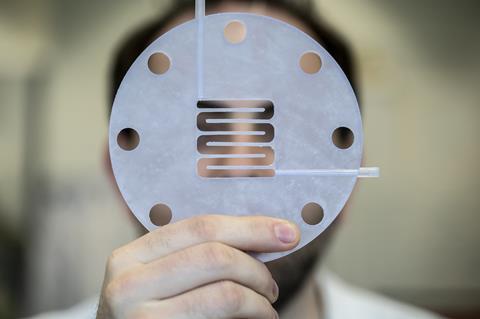
One of the main challenges is the time it takes to get the system running. With non-microbial systems, you coat your electrodes, stick them in the reactor and you can run the operation. But bacteria are alive, you first need to make them happy and set up the right conditions for them to grow. Ultimately, they decide how fast they will grow, coat the electrodes and convert CO2. And their growing speed is a serious limitation, they really need to speed up. Another point is the conversion rate. We are at about 300 Amps per square metre, that corresponds to how many electrons bacteria can take up to convert CO2 to product. The target for economic liability lies between 500 and 1000, but optimisations have only just started and I’m confident we will get there.
Then there are other challenges like scaling up the reactors; creating better 3D electrodes with 3D printing for better fluid dynamics and a place for the bacteria to grow in; and continuous extraction of product to prevent high concentrations that may be toxic to the bacteria. Clearly, we need many different areas of expertise.’
In an opinion in Cell (see box below), you and your colleague Thomas Burdyny said that the low-hanging fruit for improving MES is non-biological.
‘What we meant was: not many people had worked on reactor design, which is more abiotic. Working on bacteria is important, but if you don’t look at them in systems that make sense, you’re not investigating the right thing, in my opinion. That doesn’t mean there are no biological challenges left. We should look at both biological and abiological problems; you cannot study one without the other.’
‘Optimisations have only just started, so I’m confident we will get to economic liability’
What does the future of MES look like?
‘That depends on who you ask. My strategy has been to develop the technology while focusing on one particular high-value product, in our case hexanoic acid. This is currently extracted from palm and coconut oil, where it makes up less than one percent of all the oils, so it’s not a very sustainable process. If you can make hexanoic acid using CO2 and renewable energy, then it could become a platform chemical with applications in the development of plasticisers, lubricants, flavouring agents, pharmaceuticals, even fuels if you want. My vision is to start with one product and really develop it to the fullest extent, then you can branch out to different products.
Others are developing single cell proteins, cells consisting of sixty or seventy percent protein, which would be a very interesting food source. At the moment, there are a few companies that make these cells, like Deep Branch here in Delft or Solar Foods from Finland who make food for human consumption.’
You are a keynote speaker at the European Congress on Biotechnology. What will be your message?
‘I will share the developments within our group since we started and the projects we have taken on. We just patented a new reactor design with which we reach the highest productivity ever reported, in line with the volumetric productivity of syngas fermentation. This realisation gives us a nice way forward and motivation that MES can be applied and scaled up. A PhD student of mine was the first to develop a method to quantify the number of microorganisms in a microbial electrosynthetic system at any given time, which was not possible before. In fermentation systems, you work in suspension where quantification is a lot easier, but we work with biofilms in closed systems from which you can’t really take samples. This newly developed method is very useful, especially since we can now determine microbiome kinetics in MES systems for the first time. That gives us another layer of insight into how bacteria behave, and if and how they are limiting.
Overall, the message will be that we are moving in the right direction, but there are still some challenges left. I hope to inspire people to use these methods to describe and characterize their systems too, because I think it can be very insightful to have these insights from different research groups.’

MES and outstanding questions
In an opinion article in Cell, Ludovic Jourdin and his colleague Thomas Burdyny posed several outstanding questions for the microbial electrosynthesis field. It has been about 3,5 years since its publication. Does he think we’re any closer to the answers to these questions?
- What is really limiting MES? Can a biofilm effectively sustain current densities in the order of 100 mA cm−2 and higher?
‘We did some work on this and are now at 35 mA cm-2, which is already a big improvement from what it was [10 mA cm-2, ed.].’
- Can a general multi- and cross-scale model be built and used as a prediction tool for MES and as a tool for rational scale-up?
‘We and other groups have done work on the multi-scale models, so yes, we believe it’s possible.’
- Can extremophiles be evolved in the laboratory or enriched from natural and/or anthropogenic environments to achieve high MES rates?
‘Some colleagues in India and China are investigating this, I haven’t done this kind of work myself yet.’
- For reactor design, what inspiration can come from both large-scale fermentation reactors and large-scale electrochemical reactors? Should MES be scaled by volume or by number?
Jourdin grins: ‘To start with the last one, it’s purposefully a bit controversial. There are a lot of colleagues of mine who are scaling up by volume, more similar to a fermenter. I belong to the other school of thought, scaling up by number, so scaling up like an electrochemical system. In these systems, a major part of the costs consists of electricity costs, which are influenced by the distance between the cathode and the anode. In an electrochemical system, you stack up the reactors, so the electrodes are closer to each other. So in my opinion, this is the better way.
For the reactor design, we ourselves have made some progress with the patented design I will talk about at the ECB, but also the colleagues in the “scale up by volume” field have made some good progress.
So in the end, after 3,5 years, there certainly has been some progress. You always want more, but we’re going in the right direction. Maybe we have been a little too ambitious, but I believe you can’t make progress without ambition.’













Nog geen opmerkingen