Hot plasmas are better than cold plasmas for cracking ammonia for green hydrogen. This is what Antwerp researchers report in the Chemical Engineering Journal.
Ammonia is a potential carrier for storing green hydrogen. But if you want to extract the hydrogen from the ammonia, you have to crack it. This can be done thermally, but this goes against the sustainable idea of green hydrogen. Another option is plasma. ‘Cold plasmas are usually used for this’, explains Annemie Bogaerts, plasma professor at the University of Antwerp. ‘But in cold plasmas, the electrons have too much energy to dissociate ammonia efficiently.’ Bogaerts and her colleagues, Igor Fedirchyk, Ivan Tsonev and Rubén Quiroz Marnef, therefore decided to turn to hot plasmas (see box below), in part because not a great deal of research had been done on them.
‘Hot plasmas have better energy efficiency, we already know that from other applications’, explains Bogaerts. ‘In hot plasmas, the gas temperature is higher, but at the same time the electron energy is lower. Because of the high plasma temperature, thermal reactions take place in addition to the normal electron activation.’ The researchers found that the conversion of NH3 to nitrogen and hydrogen in these hot plasmas is mainly due to thermal reactions.
Lucky
The many years of experience that Bogaerts’ group already has in other applications of plasma – such as CO2 conversion, CH4 conversion and NOx formation from air for sustainable fertiliser production – helped in this research. We tested a wide range of conditions with four different plasma reactors,” says Bogaerts. These gave better results than previously published studies. ‘Many groups only have one plasma reactor, but we are lucky enough to have several. This allows us to use different types of hot plasmas and find out what works best.’
Nevertheless, there is still a lot to gain, according to the Antwerp professor. ‘At higher powers, we see better results, but then the plasma gets too hot and damages the walls of the reactors. We are therefore looking for new materials.’ Another problem is that not all of the gas goes through the plasma, some of it bypasses it because the plasma is mainly in the centre of the reactor, so not all of the ammonia is converted into hydrogen.
Read more below the image
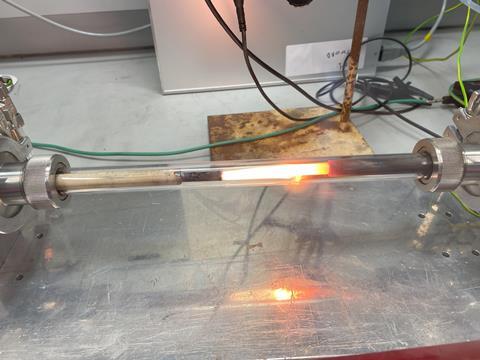
Industry
Challenges remain, but the principle has been demonstrated. Bogaerts would now like to see this application of plasma technology find its way into industry. ‘Yes, plasmas are currently less energy-efficient than thermal catalysis for ammonia conversion, but an important detail is that we do not need catalysts, which reduces the cost.’ She is therefore convinced that plasma has great potential for the chemical industry. ‘Especially in terms of sustainability: plasma works purely on electricity and you can easily switch it on and off.’ This makes it compatible with the fluctuating power supply that comes with green electricity: if there is less available for a while, you can simply switch off a reactor.
Although this research only studied plasma without a catalyst, Bogaerts also collaborates with the group of Johan Martens, professor of surface chemistry and catalysis at KU Leuven. ‘They are doing a lot of research on catalysis and are looking for cheaper catalysts for cracking ammonia’, explains Bogaerts. ‘Together we are looking at the combination of plasma and catalysis, and his group is also building models to calculate the total cost.’
The ammonia is first converted by thermal catalysis, then the conversion is increased to over 99% using our plasma reactors. ‘We have submitted a paper on this, in which you can see that the combination of catalysis and plasma requires less catalyst than the purely catalytic process.’
All in all, Bogaerts is hopeful that plasma can revolutionise the chemical industry for the electrification of all kinds of chemical processes.
Fedirchyk, I. et al. (2024) Chem. Eng. J. 499, DOI: 10.1016/j.cej.2024.155946
Hot plasma vs. cold plasma
There are different types of plasma, each with its own advantages and disadvantages. Based on the temperature of the gas, plasmas can be divided into cold and hot plasmas. In cold plasmas, the gas remains close to room temperature and is activated by reactions with electrons. This also happens in hot plasmas, but the gas itself is also heated. In cold plasmas, the electron temperature is much higher (in the order of 10,000 - 100,000 °C), leading to electronic excitation, ionisation and dissociation of the gas, whereas in hot plasmas, the electrons are typically ‘only’ 10,000 °C and are therefore more likely to lead to vibrational excitation of the gas. This is a more energy-efficient way of splitting the gas molecules, which partly explains why hot plasmas are more energy-efficient. Moreover, this vibrational excitation also leads to heating of the gas (through a process called vibrational-translational relaxation), which can also raise the gas temperature itself to around 3,000 °C or more. Therefore, in addition to electron activation, thermal reactions are also important, which partly explains the better performance. However, because the gas temperature is still lower than the electron temperature, hot plasmas are still in ‘non-thermal equilibrium’, allowing thermodynamically difficult reactions to proceed at milder temperature and pressure conditions than ordinary classical processes.













Nog geen opmerkingen